TO THE EDITOR:
Erdheim-Chester disease (ECD) is a non-Langerhans cell histiocytosis characterized by tissue infiltration by foamy CD68+ CD1a− histiocytes.1,2 ECD has a putative neoplastic and inflammatory nature. The neoplastic hypothesis is supported by the clonality of the infiltrating histiocytes, which harbor mitogen-activated protein kinase pathway mutations, of which BRAFV600E is the most common.3-5 Immune-mediated mechanisms contribute to histiocytic infiltration through a proinflammatory cytokine–chemokine network.6,7 Different treatments targeting these pathogenic mechanisms are considered first-line approaches for ECD, namely interferon-α (IFN-α), BRAFV600E, and MEK inhibitors. Their efficacy, however, is variable, depending on underlying mutations and organ involvement, and frequently limited by remarkable toxicity.8-16
The mammalian target of rapamycin (mTOR) regulates cell growth, proliferation and apoptosis, and modulates immune responses. mTOR inhibitors (mTORi’s) (eg, sirolimus, everolimus) are used to treat several neoplastic and inflammatory conditions and prevent allograft rejection.17,18 We demonstrated mTOR pathway activation in ECD lesions and provided preliminary evidence of the efficacy of a sirolimus- and prednisone-based regimen in a trial involving 10 patients.19
We report here the long-term outcomes of 20 consecutive patients with ECD treated with mTORi’s; the study includes an extended follow-up of the 10 patients enrolled in our previous trial19 and 3 patients reported in a case series,20 plus 7 new cases. Eligibility criteria are detailed in supplemental Methods (available on the Blood Web site).
The main patient characteristics are described in supplemental Table 1. Eighteen patients had a biopsy-proven diagnosis; the remaining 2 (patients 18 and 20) were diagnosed based on typical imaging findings. BRAFV600E mutation was found in 8 out of 16 tested patients. Retroperitoneal involvement was detected in 17 patients; 13 of them had hydronephrosis, requiring ureteral stenting or nephrostomy in 4 cases. Three patients had stage 4 chronic kidney disease according to the National Kidney Foundation classification. Large-vessel and bone involvement were detected respectively in 14 and 16 patients, whereas 6 showed central nervous system (CNS) involvement on magnetic resonance imaging (MRI). Cardiac disease was detected in 8 patients (13 were studied using cardiac MRI).21,22 Interstitial lung disease was radiologically found in 6 patients. Other manifestations (endocrine, skin, and soft-tissue infiltration) were also observed (supplemental Table 1).
Fourteen patients received mTORi’s as first-line therapy (supplemental Table 1). Sirolimus was prescribed to 15 patients at a single oral dose of 2 mg/day and subsequently titrated to reach blood levels of 8 to 12 ng/mL (in the 10 patients included in our previous trial,19 prednisone was combined). The remaining 5 patients received everolimus monotherapy at a dose of 0.75 mg twice daily, titrated to reach blood levels of 8 to 12 ng/mL.
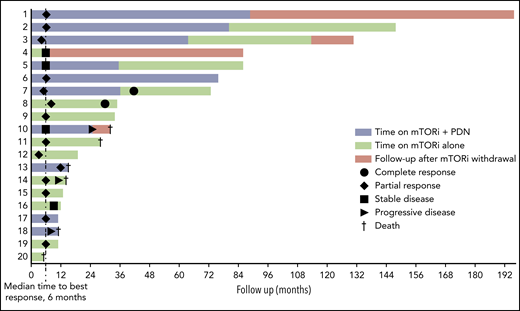
Response to treatment and survival are described in Figure 1. Response was assessed using clinical evaluation, computed tomography (CT), MRI, bone scintigraphy, and positron emission tomography (PET) CT. Radiologic and metabolic responses were defined according to RECIST and PERCIST criteria (supplemental Methods).23,24 The first assessment of response was performed at months 4 to 6.
Swimmer plot of response to treatment and survival. Patients 18 and 20 had a nonbiopsy-proven diagnosis. PDN, prednisone.
Swimmer plot of response to treatment and survival. Patients 18 and 20 had a nonbiopsy-proven diagnosis. PDN, prednisone.
The median time on treatment was 21.5 months (interquartile range [IQR], 11.5-71.5); the median follow-up was 30 months (IQR, 12.5-78) in all patients and 54 months (IQR, 13-86) in those who were alive at last follow-up. The median time to best response was 6 months (Figure 1).
Complete response (CR; defined as metabolic or radiologic, ie, either complete disappearance of FDG-uptake on PET or complete lesion regression on CT/MRI) was achieved in 2 patients. Patient 7 had bone, cardiac, large-vessel, and retroperitoneal involvement and achieved metabolic CR at month 41. Patient 8, who had bone, large-vessel, and retroperitoneal involvement, achieved metabolic CR at month 30.
A sustained partial response (PR; defined as either partial improvement of lesions on PET and/or >30% regression at CT/MRI, whichever proved best) was achieved in 11 patients (Figure 2; supplemental Figure 1). Nine of them were still alive at last follow-up, whereas 2 (patients 11 and 13) died due to non–ECD-related comorbidities (rhabdomyolysis and small-cell lung cancer). Stable disease was achieved and maintained until last follow-up in 3 patients.
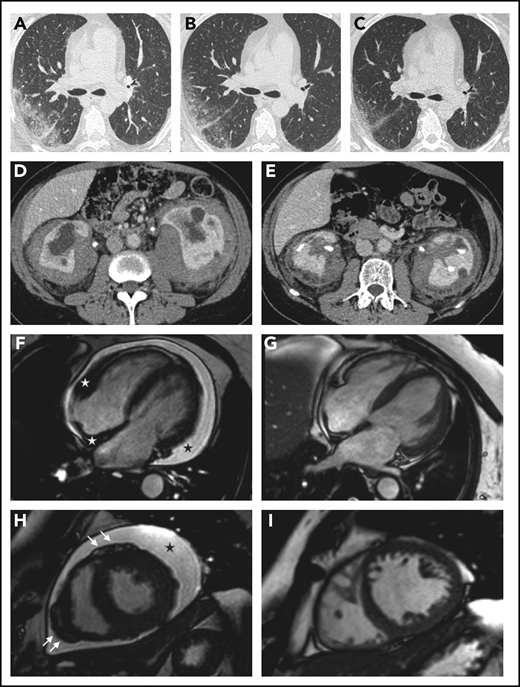
Organ responses. (A-C) Chest high-resolution CT scans in patient 12 show progressive regression of ECD-related lung disease (the scans were taken respectively before treatment and at months 6 and 12 after the beginning of therapy with everolimus) (axial view). (D-E) Contrast-enhanced abdominal CT scans in patient 8 show marked regression of perirenal infiltration with consequent improvement of hydronephrosis and calycectasia (axial view). (F-I) Cardiac magnetic resonance (cine frame from steady-state free precession sequence) in a patient (#19) with cardiac involvement; the scans were taken at baseline (F,H) and after 1 year of treatment (G,I). (F) Circumferential pericardial effusion (black star) and pathologic soft tissue in the right atrioventricular groove and the right side of the interatrial septum (white stars). Arrows (H) indicate nodular thickening of the visceral pericardium. Pericardial effusion and infiltrative lesions almost completely disappeared (G,I; scans after treatment).
Organ responses. (A-C) Chest high-resolution CT scans in patient 12 show progressive regression of ECD-related lung disease (the scans were taken respectively before treatment and at months 6 and 12 after the beginning of therapy with everolimus) (axial view). (D-E) Contrast-enhanced abdominal CT scans in patient 8 show marked regression of perirenal infiltration with consequent improvement of hydronephrosis and calycectasia (axial view). (F-I) Cardiac magnetic resonance (cine frame from steady-state free precession sequence) in a patient (#19) with cardiac involvement; the scans were taken at baseline (F,H) and after 1 year of treatment (G,I). (F) Circumferential pericardial effusion (black star) and pathologic soft tissue in the right atrioventricular groove and the right side of the interatrial septum (white stars). Arrows (H) indicate nodular thickening of the visceral pericardium. Pericardial effusion and infiltrative lesions almost completely disappeared (G,I; scans after treatment).
Three patients progressed despite treatment. Patient 14 achieved PR (month 6) but died of progressive pulmonary ECD (month 14). Patient 18 had severe CNS involvement; he refused to take IFN-α, switched to methotrexate but resumed sirolimus 1 month later because of poor methotrexate tolerability, and died at month 11. Patient 10, after initial stabilization, had cardiac and retroperitoneal progression; she switched to vemurafenib (month 25) but died a few months later (month 32). Patient 20 was not assessable for response because he died of ischemic heart disease at month 5. Overall, the median progression-free survival was 26.5 months (IQR, 11.5-82.5) (supplemental Figure 2); progression-free survival rates at 12 and 24 months were 83% and 66%, respectively. The overall survival rate was 88% at 1 year and 54% at 3 and 5 years. The overall mortality was 30% (median follow-up, 30 months).
The best objective responses were observed at the retroperitoneal, cardiac, and large-vessel levels. Retroperitoneal lesions partially or completely regressed in 9 out of 17 patients (53%) and stabilized in 7 patients. Two of the 3 patients who underwent ureteral stenting became stent-free. Cardiac lesions partially regressed on MRI in 6 out of 8 patients (75%). Six patients remained free of pericardial effusion over the entire follow-up, whereas patient 10 progressed. Large-vessel lesions improved in 11 out of 14 patients (79%).
Bone disease remained stable or slightly improved on bone scintigraphy in all patients. CNS lesions partially regressed in 2 out of 6 patients (33%), stabilized in 3 patients, and progressed in 1 patient (#18). Lung involvement improved in 2 out of 6 patients and stabilized in 2 patients; patient 14 died of pulmonary involvement (supplemental Table 2).
Treatment was well tolerated in most cases. The most common adverse events were worsening of diabetes (15%) and dyslipidemia (10%) (supplemental Table 3). Two patients temporarily discontinued treatment due to drug-related toxicity (infectious panniculitis, lymphocytic alveolitis). One of them discontinued sirolimus and relapsed 2 years later with new-onset pleural lesions. Patient 4 discontinued treatment of acute kidney failure of uncertain origin; she later started IFN-α, maintaining disease stabilization.
In this study of 20 patients with ECD, we observed efficacy and good tolerability of mTORi’s. Ten patients were included in our sirolimus plus prednisone study,19 and their extended follow-up is reported herein. Prompted by the encouraging trial results, we treated 10 additional patients with mTORi’s; in these cases, glucocorticoids were avoided, because accumulating evidence had questioned their therapeutic role in ECD.8 The high response rate observed after mTORi monotherapy further supports the view that they are efficacious per se.
Overall, mTORi’s induced CT/MRI or metabolic responses in 13 out of 20 patients (65%) and stable disease in 3 patients (15%). Most responses were sustained, and most patients were still on treatment at last visit. The overall mortality was higher than in other series, although our patients suffered from severe comorbidities, and half the deaths were ECD unrelated. Drug-related side effects were limited.
The therapeutic approach to ECD is quite complex. IFN-α is the traditional first-line therapy, but BRAFV600E and MEK inhibitors are candidate first-line options, especially for severe cases.8-16,25 However, their use is limited by side effects and high costs. ECD patients that can benefit from mTORi’s might include those with nonsevere disease or those who have no access or contraindications to targeted therapies or IFN-α. Since mTORi’s induced responses in both BRAFV600E and wild-type patients, they could be used irrespective of the BRAF status.
In summary, mTORi’s, even if used as monotherapy, represent a valid alternative to conventional ECD treatments, as they induce high response rates and are generally well tolerated.
The online version of this article contains a data supplement.
Authorship
Contribution: F. Pegoraro and A.V. designed the study, analyzed the data, and wrote the manuscript; V.M., F. Peyronel, P.J.W., R.M.R., P.R., and E.F.H.v.B. followed the patients and collected the data; E.S. performed tumor sequencing; A.A.P. and T.R.H. reviewed the imaging studies and generated the imaging figures; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Augusto Vaglio, Dipartimento di Scienze Biomediche, Sperimentali e Cliniche “Mario Serio”, Università di Firenze, Viale Pieraccini 6, 50139 Firenze, Italy; e-mail: augusto.vaglio@unifi.it.
REFERENCES
Author notes
F. Pegoraro and V.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal