To the editor:
Erdheim-Chester disease (ECD) is a rare non-Langerhans cell histiocytosis (<1000 cases reported in the literature), characterized by tissue infiltration by CD68+ CD1a− “foamy” histiocytes. ECD commonly causes long bone osteosclerosis, retroperitoneal (periaortic and perirenal) fibrosis, central nervous system (CNS) lesions, but also involves the lung, the skin, and various endocrine axes.1 Cardiovascular manifestations are also common (∼40% of the cases) and include infiltration of the myocardium (eg, pseudotumoral atrial masses), the pericardium (eg, pericarditis sometimes complicated by tamponade), and the aorta, with the typical aspect of “coated aorta.”2,3 Patients with ECD with cardiovascular involvement are reported to have a poorer prognosis1,4,5 and are therefore usually treated aggressively, but systematic studies are lacking. It is also unclear whether cardiovascular involvement only detected by imaging studies actually influences prognosis, and whether the purported adverse prognosis is due to cardiovascular lesions per se or to other factors.
With the aim of better characterizing ECD-related cardiovascular lesions and their potential prognostic impact, we conducted a cross-sectional study on 23 consecutive patients with ECD using a standardized protocol of cardiac magnetic resonance imaging (MRI) and magnetic resonance (MR) angiography of the thoracic aorta and epiaortic arteries. MRI was performed using a 1.5-T MR Scanner (Gyroscan Intera; Philips Medical System, Best, The Netherlands) with a maximum gradient capability of 30 mT/m and maximum slew rate of 150 mT/ms. MR acquisition was triggered by electrocardiography and included functional study by means of balanced steady-state free precession (b-SSFP) cine-MR images in the short-axis planes, and vertical long-axis and horizontal long-axis planes; morphologic evaluation was performed with T2-weighted (T2w) images and short time inversion recovery T2w images in order to suppress fatty tissue signal and emphasize tissue and myocardial edema/inflammation. Finally, 3-dimensional gradient echo/segmented inversion recovery T1-weighted (T1w) sequences were performed for the evaluation of delayed enhancement 10 minutes after intravenous gadolinium-diethylenetriamine pentaacetic acid injection (dose: 0.2 mmol/kg).
All patients were referred to or diagnosed at the Nephrology Unit of Parma University Hospital between January 2007 and October 2015; of them, 7 were included in a previous trial6 and 1 was described in a case report.7 They all underwent cardiac MRI at first evaluation at our center; in 16 of them, cardiac MRI was performed at the time of diagnosis, whereas in the remaining 7, MRI was performed after a median time from diagnosis of 72 months (range 19-180). All patients had biopsy-proven ECD. The study protocol was approved by the local ethics committee; all patients signed an informed consent.
Twenty patients (87%) were men; the median age at diagnosis was 48 years (range 22-72). Fourteen patients (61%) had traditional cardiovascular risk factors (eg, diabetes, dyslipidemia), and 5 patients had overt cardiovascular disease (eg, coronary artery disease, peripheral arterial disease, atherosclerotic aortic aneurysm) prior to ECD diagnosis (supplemental Table 1, available on the Blood Web site). Table 1 illustrates the main sites involved by the disease. Ten patients (43%) had MRI evidence of cardiac involvement, with myocardial involvement in 9 and pericardial involvement in 9. Six patients had thoracic large-vessel involvement together with cardiac lesions, whereas only 1 patient had thoracic aorta involvement without cardiac disease (Table 1). MRI revealed peculiar patterns of myocardial involvement (Figure 1): most patients with cardiac disease had right atrial involvement, usually in the form of an atrial pseudotumoral mass, mainly involving the posterior atrial wall and often protruding into the atrium; another common lesion was the infiltration of the right atrioventricular sulcus, where the tissue usually surrounded or infiltrated the right coronary artery. However, none of the 7 patients with right pericoronary infiltration had ECD-related ischemic cardiac lesions. Unlike other infiltrative disorders, ECD did not cause diffuse infiltration of the myocardium, a finding in line with the usually normal systolic or diastolic functions observed by MRI or echocardiography (Table 1; supplemental Table 1; supplemental Video 1). Pericardial infiltration/thickening was common, often accompanied by pericardial effusion (Figure 1; supplemental Video 1) leading to tamponade in 2 cases and requiring pericardiocentesis in 3 cases. Unlike in other reports,2 we found no MRI evidence of ECD-related valvular disease. Thoracic large-artery involvement was generally characterized by perivascular thickening of the thoracic aorta and the origin of the epiaortic arteries (supplemental Figure 1), but no luminal narrowing or aneurysms were detected.
Main demographic, clinical, and cardiac MRI characteristics of the 23 patients with ECD included in the study
| Pt. no. . | Age (y), sex . | TTD (mo) . | Extracardiac involvement . | Myocardial lesions on MRI . | Pericardial lesions on MRI . | MRI EF % . | Thoracic vessel involvement on MRI . | BRAF status . |
|---|---|---|---|---|---|---|---|---|
| 1 | 46, M | 55 | B, RP, HPG, O | Right atrial pseudomass | Pericardial thickening | 68 | AA, AArch, BT, LSA, LCCA | Wild type |
| Right AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion | |||||||
| 2 | 47, M | 117 | B, RP, HPG, S | Right atrial pseudomass; both AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion with tamponade | 68 | AA, LSA | V600E |
| 3 | 42, F | 190 | B, RP, HPG, L, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 61 | — | V600E |
| Mild pericardial effusion | ||||||||
| 4 | 49, F | 96 | O, S | — | — | 69 | — | Wild type |
| 5 | 71, M | 1 | RP, O | — | — | 51 | — | NA |
| 6 | 60, M | 5 | B, RP, HPG, CNS, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 63 | — | V600E |
| Mild pericardial effusion | ||||||||
| 7 | 43, M | 78 | B, CNS, S | — | — | 58 | — | NA |
| 8 | 41, M | 3 | RP | Right atrial pseudomass; right AV sulcus infiltration | Mild pericardial effusion | 65 | — | Wild type |
| 9 | 25, M | 15 | RP, O | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion with tamponade | 60 | AA, AArch, SVC, IVC, PA, PV | V600E |
| 10 | 22, M | 39 | B, HPG, CNS, S | — | — | 70 | — | V600E |
| 11 | 72, M | 1 | B, RP | — | — | 60 | — | V600E |
| 12 | 32, M | 12 | B, HPG, CNS, L, S | Right atrial pseudomass; right AV sulcus and auricolar infiltration and right pericoronary infiltration | Pericardial thickening | 66 | SVC | V600E |
| Mild pericardial effusion | ||||||||
| 13 | 59, M | 58 | B, CNS | — | — | 64 | — | NA |
| 14 | 70, F | 30 | B, RP, HPG, L, O | — | Pericardial thickening | 54 | AArch, BT, LCCA, LSA | V600E |
| Mild pericardial effusion | ||||||||
| 15 | 48, M | 216 | B (lytic and sclerotic), RP, HPG, L, S | Focal right atrial thickening | — | 55 | AA | Wild type |
| 16 | 50, M | 11 | B, RP | — | — | 71 | DA | Wild type |
| 17 | 44, M | 68 | B, RP, HPG, L | — | — | 55 | — | NA |
| 18 | 45, M | 107 | B, HPG | — | — | 53 | — | V600E |
| 19 | 55, M | 4 | B, RP, HPG | — | — | 58 | — | NA |
| 20 | 69, M | 36 | B, RP, HPG, S | — | — | 55 | — | V600E |
| 21 | 56, M | 30 | RP | — | — | 53 | — | V600E |
| 22 | 47, M | 36 | B, RP, HPG, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 58 | — | V600E |
| Mild pericardial effusion | ||||||||
| 23 | 48, M | 180 | RP, S | — | — | 69 | — | Wild type |
| Pt. no. . | Age (y), sex . | TTD (mo) . | Extracardiac involvement . | Myocardial lesions on MRI . | Pericardial lesions on MRI . | MRI EF % . | Thoracic vessel involvement on MRI . | BRAF status . |
|---|---|---|---|---|---|---|---|---|
| 1 | 46, M | 55 | B, RP, HPG, O | Right atrial pseudomass | Pericardial thickening | 68 | AA, AArch, BT, LSA, LCCA | Wild type |
| Right AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion | |||||||
| 2 | 47, M | 117 | B, RP, HPG, S | Right atrial pseudomass; both AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion with tamponade | 68 | AA, LSA | V600E |
| 3 | 42, F | 190 | B, RP, HPG, L, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 61 | — | V600E |
| Mild pericardial effusion | ||||||||
| 4 | 49, F | 96 | O, S | — | — | 69 | — | Wild type |
| 5 | 71, M | 1 | RP, O | — | — | 51 | — | NA |
| 6 | 60, M | 5 | B, RP, HPG, CNS, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 63 | — | V600E |
| Mild pericardial effusion | ||||||||
| 7 | 43, M | 78 | B, CNS, S | — | — | 58 | — | NA |
| 8 | 41, M | 3 | RP | Right atrial pseudomass; right AV sulcus infiltration | Mild pericardial effusion | 65 | — | Wild type |
| 9 | 25, M | 15 | RP, O | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Massive pericardial effusion with tamponade | 60 | AA, AArch, SVC, IVC, PA, PV | V600E |
| 10 | 22, M | 39 | B, HPG, CNS, S | — | — | 70 | — | V600E |
| 11 | 72, M | 1 | B, RP | — | — | 60 | — | V600E |
| 12 | 32, M | 12 | B, HPG, CNS, L, S | Right atrial pseudomass; right AV sulcus and auricolar infiltration and right pericoronary infiltration | Pericardial thickening | 66 | SVC | V600E |
| Mild pericardial effusion | ||||||||
| 13 | 59, M | 58 | B, CNS | — | — | 64 | — | NA |
| 14 | 70, F | 30 | B, RP, HPG, L, O | — | Pericardial thickening | 54 | AArch, BT, LCCA, LSA | V600E |
| Mild pericardial effusion | ||||||||
| 15 | 48, M | 216 | B (lytic and sclerotic), RP, HPG, L, S | Focal right atrial thickening | — | 55 | AA | Wild type |
| 16 | 50, M | 11 | B, RP | — | — | 71 | DA | Wild type |
| 17 | 44, M | 68 | B, RP, HPG, L | — | — | 55 | — | NA |
| 18 | 45, M | 107 | B, HPG | — | — | 53 | — | V600E |
| 19 | 55, M | 4 | B, RP, HPG | — | — | 58 | — | NA |
| 20 | 69, M | 36 | B, RP, HPG, S | — | — | 55 | — | V600E |
| 21 | 56, M | 30 | RP | — | — | 53 | — | V600E |
| 22 | 47, M | 36 | B, RP, HPG, O, S | Right atrial pseudomass; right AV sulcus infiltration and right pericoronary infiltration | Pericardial thickening | 58 | — | V600E |
| Mild pericardial effusion | ||||||||
| 23 | 48, M | 180 | RP, S | — | — | 69 | — | Wild type |
—, absent; AA, ascending aorta; AArch, aortic arch; AV, atrioventricular; B, bone; BT, brachiocephalic trunk; DA, descending aorta; EF, ejection fraction; F, female; HPG, hypothalamic-pituitary gland axis; IVC, inferior vena cava; L, lung; LCCA, left common carotid artery; LSA, left subclavian artery; M, male; NA, not available; O, orbits; PA, pulmonary artery; Pt, patient; PV, pulmonary vein; RP, retroperitoneum; S, skin; SVC, superior vena cava; TTD, time from symptom onset to diagnosis.
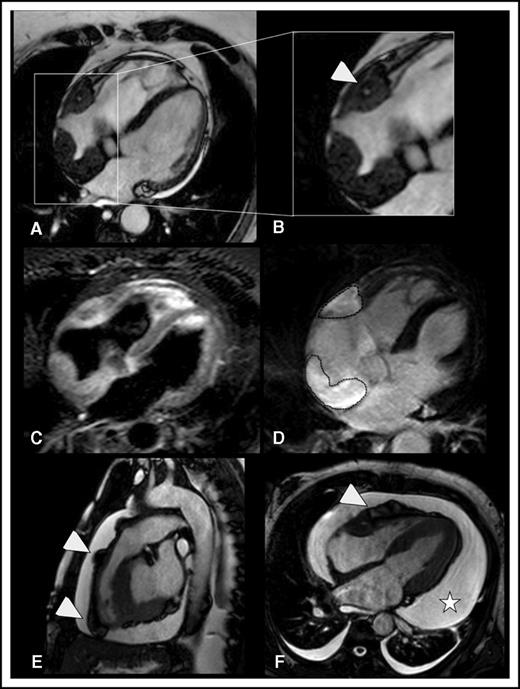
Myocardial and pericardial involvement in ECD. (A-D) Myocardial lesions. ECD typically affects the myocardium by infiltrating the posterior wall of the right atrium, forming soft tissue density masses with smooth surface, which appear hypointense in b-SSFP sequences (the lesions are framed in A). This b-SSFP hypointense infiltration also develops in the right atrioventricular groove, where it encases the right coronary artery (arrowhead in B). T2w image with signal fat suppression shows that there might be diffuse slight hyperintensity within the soft tissue, which usually corresponds to edema (C). In inversion recovery T1w sequences, late enhancement of the lesional soft tissue likely reflects disease activity/inflammation (dotted lines in D). (E-F) Pericardial lesions. ECD-related infiltration of the pericardium causes soft tissue density nodules arising from the visceral pericardial sheets, in association with pericardial effusion. b-SSFP sequences (E-F) allow optimal visualization of the nodules that appear hypointense as compared with the hyperintense pericardial effusion. Arrowheads indicate soft tissue pericardial nodules in the right ventricle outflow tract (E) and right ventricle free wall (F). The pericardial nodules are associated with conspicuous circumferential pericardial effusion (star in F). (A-D,F) Four-chamber view (a plane that allows comprehensive evaluation of myocardium and pericardium). (E) Sagittal view (the best plane to depict the right ventricle ouflow tract).
Myocardial and pericardial involvement in ECD. (A-D) Myocardial lesions. ECD typically affects the myocardium by infiltrating the posterior wall of the right atrium, forming soft tissue density masses with smooth surface, which appear hypointense in b-SSFP sequences (the lesions are framed in A). This b-SSFP hypointense infiltration also develops in the right atrioventricular groove, where it encases the right coronary artery (arrowhead in B). T2w image with signal fat suppression shows that there might be diffuse slight hyperintensity within the soft tissue, which usually corresponds to edema (C). In inversion recovery T1w sequences, late enhancement of the lesional soft tissue likely reflects disease activity/inflammation (dotted lines in D). (E-F) Pericardial lesions. ECD-related infiltration of the pericardium causes soft tissue density nodules arising from the visceral pericardial sheets, in association with pericardial effusion. b-SSFP sequences (E-F) allow optimal visualization of the nodules that appear hypointense as compared with the hyperintense pericardial effusion. Arrowheads indicate soft tissue pericardial nodules in the right ventricle outflow tract (E) and right ventricle free wall (F). The pericardial nodules are associated with conspicuous circumferential pericardial effusion (star in F). (A-D,F) Four-chamber view (a plane that allows comprehensive evaluation of myocardium and pericardium). (E) Sagittal view (the best plane to depict the right ventricle ouflow tract).
Six of the 10 patients with cardiac MRI abnormalities had cardiovascular symptoms during their disease course: in particular, patients 1, 2, and 9 had peripheral edema and dyspnea, and patient 2 also chest pain, due to pericarditis; patient 14 presented with peripheral edema and dyspnea due to acute cardiac failure; patient 6 presented with palpitations and dyspnea secondary to atrial flutter; and patient 15 presented with angina and syncope likely secondary to atherosclerotic, non-ECD-related, coronary artery disease. Cardiovascular symptoms were also found in patients without cardiac MRI abnormalities, due to atherosclerosis-related ischemic heart disease (patients 13 and 17) and uremic pericarditis (patient 19) (supplemental Table 1). Only 1 patient (patient 6) underwent cardiac electrophysiology study (when radiofrequency ablation was performed for atrial flutter), but we could not retrieve the results of this study.
The patients received different treatments, mainly sirolimus and prednisone,6 interferon-α, vemurafenib, and chemotherapy; there were no significant differences in the distribution of the different therapies between patients with and without cardiac MRI abnormalities (data not shown). Eight patients (7 of whom had cardiac MRI abnormalities) underwent follow-up cardiac MRI: the lesions remained stable in 4 patients (2 treated with interferon-α, 1 treated with prednisone, and 1 treated with vemurafenib), partially regressed in 2 (both treated with sirolimus and prednisone) (supplemental Video 1), and progressed in 1 (sequentially treated with sirolimus and vemurafenib) (supplemental Table 2). Three patients died, 1 in the cardiac group and 2 in the noncardiac group. No significant differences in overall survival were found between the 2 groups (log-rank test P = .57), although it must be acknowledged that the mortality rate was low (supplemental Figure 2). Progression-free survival, defined as time from remission to disease progression, was also similar (log-rank test P = .63) (supplemental Figure 2). The demographic characteristics and the frequency of organ involvement did not differ between the 2 groups, although most manifestations tended to be more frequent in the group with cardiac abnormalities (supplemental Table 3). When we investigated disease extension by calculating the number of involved sites, we found that patients with cardiac MRI abnormalities had a significantly higher number of (extracardiac) involved sites than those without cardiac lesions (P = .0097); in particular, all patients with disseminated disease (≥5 sites involved) were in the cardiac group (supplemental Figure 3). No cluster analyses have been performed in ECD, but it is known that its clinical spectrum ranges from organ-limited, asymptomatic forms to disseminated and life-threatening disease.8 These findings demonstrate that cardiac involvement (either clinical or subclinical) is associated with a more widespread disease.
In conclusion, cardiac and angio-MRI reveals peculiar patterns of cardiac and large-vessel involvement in ECD and often allows detection of clinically silent lesions. It is therefore advisable that all patients with ECD undergo cardiac and angio-MRI at the time of diagnosis, and in case of new cardiovascular manifestations or overall disease progression. In our series, MRI-detected cardiac disease did not have an impact on survival, although this finding is limited by the low mortality rate observed and should be confirmed by larger studies. However, MRI-detected cardiac involvement was clearly associated with a greater disease burden, which could therefore explain the worse outcome of patients with ECD with cardiac lesions observed in previous studies.
The online version of this article contains a data supplement.
Authorship
Contribution: D.G., A.A.P., M.D.F., and A.V. designed the study and wrote the manuscript; A.A.P., M.D.F., C.M., E.R., and L.B. performed MRI and analyzed the MRI results; G.M., A.V., and D.G. collected clinical data; A.V. and D.G. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Augusto Vaglio, Unità Operativa di Nefrologia, Azienda Ospedaliero-Universitaria di Parma, Via Gramsci 14, 43126 Parma, Italy; e-mail: augusto.vaglio@virgilio.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal