In this issue of Blood, Diamond et al provide the first consensus guidelines for the diagnosis and clinical management of patients with Erdheim-Chester disease (ECD), a rare disorder where diagnosis can be delayed and effective therapies are lacking.1
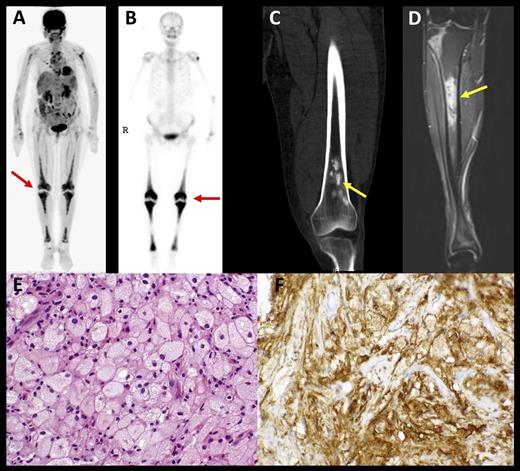
Characteristic histopathologic and radiographic findings of ECD. (A) PET and (B) 99mTc imaging demonstrating symmetric diametaphyseal radiotracer uptake in the long bones of the legs (arrows) commonly seen in ECD patients. (C) CT and (D) MRI scans revealing sclerotic lesions of the metaphyses of femur and tibia (arrows). (E) Hematoxylin-and-eosin–stained biopsy section of ECD lesion revealing lipid-laden histiocytes characteristic of ECD. (F) Immunohistochemical stain for CD68 revealing positivity of histiocytes. CT, computed tomography; PET, positron emission tomography. See Figure 1 in the article by Diamond et al that begins on page 483
Characteristic histopathologic and radiographic findings of ECD. (A) PET and (B) 99mTc imaging demonstrating symmetric diametaphyseal radiotracer uptake in the long bones of the legs (arrows) commonly seen in ECD patients. (C) CT and (D) MRI scans revealing sclerotic lesions of the metaphyses of femur and tibia (arrows). (E) Hematoxylin-and-eosin–stained biopsy section of ECD lesion revealing lipid-laden histiocytes characteristic of ECD. (F) Immunohistochemical stain for CD68 revealing positivity of histiocytes. CT, computed tomography; PET, positron emission tomography. See Figure 1 in the article by Diamond et al that begins on page 483
ECD, a rare non-Langerhans cell histiocytosis (LCH) affecting mainly adults, is characterized by the pathologic accumulation of foamy histiocytes in organs such as bone, kidneys, retroperitoneum, pituitary, brain, lungs, skin, orbits, and heart. The clinical picture ranges from asymptomatic infiltration to fulminant organ failure.2 Although first described in 1930, the etiology of ECD remains unclear. There is no standard therapy for patients with ECD and response to treatment can be variable. The prognosis of ECD is guarded with more than half of patients dying of their disease within 3 years from diagnosis.3 Large prospective trials are difficult to conduct due to the variability in the natural history and the small number of patients worldwide.
In October 2013, the ECD Global Alliance organized the first international medical symposium for ECD, where a multidisciplinary group of experts met to provide recommendations for the diagnosis and treatment of ECD. The article by Diamond and colleagues summarizes these recommendations based on the minimal available clinical data along with a consensus of those involved in that conference. Such guidelines are a double-edged sword. On the one side of the blade, they can provide a basis to standardize approaches to diagnosis, treatment, and follow-up for physicians caring for patients with a rare disease. On the other side of the blade, they are often based on limited data and do not rigorously follow standards for clinical practice guideline development.4 As noted in the Diamond et al article, the best levels of evidence were included from case reports, series, and retrospective studies with 40% of the evidence based only on expert opinion. Therefore, we should not refer to such publications as “guidelines” even with the qualifying term “consensus.” As Pilate succinctly stated without waiting for a response, “What is truth?” It is clearly a difficult question but one for which most of us will indeed stick around for the answer. Thus, Diamond et al have provided an important beginning.
First, the report highlights and helps to resolve the diagnostic difficulties that many practitioners encounter at the initial presentation of a patient with ECD. The diagnosis of ECD is usually established based on radiologic, clinical, and histopathological findings. Lipid-laden histiocytes and Touton giant cells with a strong positivity for factor XIIIa are typical for ECD. Bilateral symmetrical long bone involvement and radiologic osteosclerosis along with characteristic cardiovascular, lung, and retroperitoneal infiltrating disease are typical features of ECD (see figure).
Second, the report emphasizes the importance of the observation of recurrent BRAFV600E mutations in 50% to 100% of ECD in terms of the diagnostic work-up, and also strongly supports that ECD is a clonally derived and RAS-RAF-MEK-ERK–driven disorder.5-7 This observation and the anecdotal responses reported with the BRAF600E inhibitor, vemfurafenib, have provided the rationale for clinical testing of such pathway-directed treatments in ECD.8
Third, the proposed clinical classification of ECD as asymptomatic and symptomatic will be helpful in tailoring therapy according to disease severity. Asymptomatic ECD patients may do very well with a watch-and-wait strategy while treatment, albeit not optimal, with interferon α, does appear to improve overall survival.9 To this end, Diamond et al provide the first treatment algorithm for patients with ECD based on the information currently available. They appropriately emphasize the importance of first considering available (of which there are at least 3 ongoing) clinical trials for these patients. One of the key areas of clinical trial testing appropriately involves examining inhibition of BRAFV600E. It should be noted that although the anecdotal responses in the 3 patients with ECD were clear, the follow-up was short and there is no indication that the disease was in anyway eradicated. Nevertheless, such observations are important and can provide the foundation for trials that can now try to answer questions of dose, schedule, duration, and what patients should be included. Several warning signs exist for the use of such inhibitors. First is the relatively rapid emergence of disease resistance through a varied number of molecular mechanisms, suggesting the need to move rapidly to trials testing combination therapies. Another concern is the relatively high incidence of cutaneous malignancies in patients treated with BRAFV600E inhibitors along with other cutaneous adverse effects. The report of a patient on both BRAF and MEK inhibitors who developed a RAS-mutated pancreatic cancer deepens the need to study novel treatment approaches on carefully monitored clinical trials.10
There also remains the intriguing question of why some patients with BRAFV600E somatic, activating mutations of a hematopoietic lineage develop ECD vs LCH vs hairy cell leukemia. Is the cell context or maturational stage key to this question? And if the mutations arise in a similar precursor, what other factors direct the aberrant cell along such different neoplastic paths? Clearly, more work will be necessary and that work will require standardized diagnostic and response criteria. Such goals will also need to be applied to other histiocytic disorders, such as juvenile xanthogranulomatous disease and Rosai-Dorfman disease.
High aerial acts without a net are dangerous, but attract audiences. Bringing more attention to rare diseases like ECD will hopefully serve a similar purpose.
Conflict-of-interest disclosure: The authors declare no competing financial interests.