In this issue of Blood,Cohen Aubart et al present a large series of patients with Erdheim-Chester disease (ECD) treated with BRAF and MEK inhibitors and describe the disease trajectory in a subset of these patients after treatment withdrawal.1
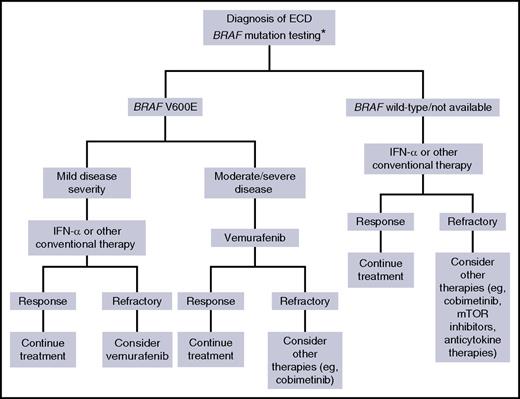
Proposed therapeutic algorithm for patients with ECD. Asterisk (*) indicates that molecular testing of non-BRAF mutations, performed using next-generation sequencing, is recommended for BRAF wild-type cases, either immediately when found to be BRAF wild-type or if there is disease progression on treatment.
Proposed therapeutic algorithm for patients with ECD. Asterisk (*) indicates that molecular testing of non-BRAF mutations, performed using next-generation sequencing, is recommended for BRAF wild-type cases, either immediately when found to be BRAF wild-type or if there is disease progression on treatment.
The past few years have built ECD into a monument to the potential of genomic research to revolutionize the understanding, management, and outcome of a rare disorder. First described as a “lipoid granulomatosis” by Erdheim and Chester in 1930, ECD was subsequently recognized as a non–Langerhans cell histiocytosis, and it was only in 1996 that diagnostic criteria were proposed.2 At that time, the treatment of ECD was empirical, mainly based on chemotherapeutic or immunosuppressive agents, and mortality rates were as high as 60% within 3 years of diagnosis.2 Subsequently, interferon-α (IFN-α) treatment was attempted,3 with a positive impact on patient outcome. However, until a few years ago, ECD was still a disease of unknown etiology, and its nature was considered to be inflammatory or reactive. The paradigm changed in 2012, when Haroche et al reported that more than half of ECD patients harbor the BRAFV600E mutation,4 and, more importantly, that the specific BRAFV600E inhibitor vemurafenib was efficacious in inducing disease remission.5 Further genomic profiling found that most BRAF wild-type patients had activating alterations of the MAPK pathway and that targeting of these alterations was also efficacious, thus corroborating the hypothesis of a clonal disorder.6 In parallel, other treatments targeting cytokines or their receptors (eg, anti-interleukin-1 receptor and anti–tumor necrosis factor α antibodies) or pathways involved in cell proliferation (eg, mammalian target of rapamycin [mTOR] inhibitors) also became available, sometimes producing encouraging responses.7-9 This exciting sequence of scientific advances not only contributed to raise awareness of the disease and boosted the reporting of new cases, but also significantly improved patient prognosis, with mortality rates dropping from 60% to 20%.9
However, despite targeted therapies (particularly vemurafenib) achieving widespread use for treatment of ECD, the studies published so far are limited by small sample sizes and short follow-up periods.
The study by Cohen Aubart et al is the first to report on vemurafenib efficacy and safety in a large ECD cohort (54 patients) with adequately long follow-up. Vemurafenib met expectations in terms of efficacy: it induced metabolic responses (assessed using 18F-fluorodeoxyglucose positron emission tomography computed tomography [18F-FDG PET-CT]) at 6 months in ∼90% of the patients, most of whom also experienced symptom improvement and tumor shrinkage. Notably, none of the patients who achieved metabolic remission had disease progression during the follow-up. These findings are important and strongly support the use of vemurafenib in ECD patients carrying the BRAFV600E mutation. However, it must be recognized that response assessment in ECD is often difficult. 18F-FDG PET-CT is one of the best surrogates for disease evaluation, but disappearance of FDG uptake does not always correspond to complete regression of the lesions, which may be because of the abundant fibrosis accompanying histiocytic infiltration. Therefore, clinical manifestations due to organ injury or compression (eg, diabetes insipidus and ureteral obstruction) often persist or only slightly improve despite excellent metabolic responses, as do neurologic abnormalities, especially those related to cerebellar atrophy or brainstem involvement.
Cohen Aubart et al’s study addresses another relevant point: vemurafenib treatment must be continued to maintain responses. Twenty of the patients enrolled in the study stopped vemurafenib (of these, 11 had achieved metabolic responses) after a median of 20 months. Treatment withdrawal was followed by relapses in 75% of the cases; the time to relapse was extremely short (median, 6 months), but, fortunately, resumption of vemurafenib worked in all patients.
Finally, the authors report on the use of the MEK inhibitor cobimetinib in a subgroup of 15 patients. The indications for cobimetinib were BRAF wild-type status, intolerance to vemurafenib, or persistent disease despite vemurafenib therapy. In these patients, assessing response to cobimetinib was a hard task, mainly because most had concurrent or previous therapies. However, of the 4 patients who received cobimetinib monotherapy, 2 achieved partial and 2 complete metabolic responses; this is certainly a positive result, which may stimulate further research into the use of cobimetinib alone. A phase 2 trial testing cobimetinib monotherapy is currently recruiting patients with histiocytic disorders who are either BRAF wildtype or BRAFV600E mutant and intolerant to, or unable to access, BRAF inhibitors (www.clinicaltrials.gov; # NCT02649972).
Thus, BRAF and MEK inhibitors appear to gain more and more traction for the treatment of ECD. However, their toxicity is considerable, and the careful assessment of treatment-related side effects is one of the major strengths of the study by Cohen Aubart et al. A wide array of adverse effects was noted in this study and in previous trials of BRAF and/or MEK inhibitors for melanoma.10 Vemurafenib causes substantial skin toxicity (eg, photosensitivity, pilar keratosis, and squamous-cell carcinomas), cardiac abnormalities (eg, QT-interval prolongation), arthralgia, and gastrointestinal side effects. Some of these manifestations (particularly gastrointestinal) can be worsened by the concurrent use of cobimetinib, which also has drug-specific side effects such as rhabdomyolysis, retinopathy, and acneiform rash.1,10 Most of these drug-related reactions were also seen in a significant proportion of Cohen Aubart et al’s patients, some of whom also developed very severe adverse effects (eg, drug reaction with eosinophilia and systemic symptoms and drug-induced vasculitis). Overall, a substantial proportion of patients (11/54, 20.4%) had to stop treatment because of toxicity.
Taken together, these data mitigate the enthusiasm initially raised by newer therapies but especially call for a careful use of these drugs and for their use in appropriate clinical settings. As also acknowledged by Cohen Aubart et al, it seems reasonable that such therapies be adopted in patients with moderate-to-severe disease or for those not tolerating other approaches, as proposed in the algorithm shown in the figure.
Other questions remain unanswered. Will these drugs remain efficacious after several years of treatment? Will ECD cells find escape mechanisms that will make them resistant to the targeted approaches? Will combinations of immunosuppressive and oncogene-directed drugs be more effective than targeted therapies alone in the treatment of ECD? The field is still open, and further research is certainly warranted to better define the pathogenic mechanisms and the best treatment approaches for ECD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal