Abstract
Immunopathogenesis of Erdheim-Chester disease (ECD), a rare non–Langerhans cell histiocytosis, is poorly known. In previous studies, various cytokines were detected in ECD lesions, presumably orchestrating lesional histiocyte recruitment. Because ECD lesions are frequently associated with systemic symptoms, we postulated that underlying global immune perturbations might also be revealed. We quantitatively analyzed 23 cytokines in serum samples obtained from a large single-center cohort of 37 patients with ECD, and studied the impact of treatment on cytokine production. IL-6, IL-12, interferon-α (IFN-α), and monocyte chemotactic protein-1 (MCP-1) levels were significantly higher in untreated patients than in controls, whereas interferon-γ (IFN-γ) inducible protein 10, IL-12, MCP-1, and IL-1 receptor antagonist were found significantly increased in IFN-α–treated patients. A biomathematical approach was used to rationalize multiparameter data, to generate new hypotheses, and identify global control pathways. Interestingly, cytokine profiles proved to be particularly stable at the individual level, and an “ECD signature” further distinguished patients from controls, based on their production of IFN-α, IL-12, MCP-1, IL-4, and IL-7. Altogether, our data underline the systemic immune Th-1–oriented perturbation associated with this condition and provide clues for the choice of more focused therapeutic agents in this rare disease with noncodified therapeutic management.
Introduction
Erdheim-Chester disease (ECD) is a rare non–Langerhans cell histiocytosis first described by Jakob Erdheim and William Chester in 1930.1 It is a systemic and heterogeneous disease mainly involving the bones, lungs, skin, retro-orbital tissues, central nervous system (CNS), pituitary gland, vessels, kidneys, retroperitoneum, and heart.2,3 The clinical course of ECD is largely dependent on the extent and distribution of disease, which may range from asymptomatic bone lesions to multisystemic, life-threatening forms with poor prognosis, especially in case of specific CNS or cardiovascular involvement.2,4 ECD diagnosis is currently based on clinical, radiologic and typical pathologic features with biopsy specimen displaying infiltration by CD68+CD1a− foamy histiocytes. Treatments for ECD include steroids, cytotoxic agents such as cladribin,5 interferon alpha-2a (interferon-α [IFN-α]),6 recombinant human interleukin-1 receptor antagonist (IL-1RA),7 thyrosine kinase inhibitors,8 biphosphonates,9 and autologous hematopoietic stem cell transplantation,10 but an optimal therapeutic strategy remains to be defined.
Our understanding of the pathogenesis of ECD is limited because the disease is very rare, and thus previous studies could only be performed in a small cohort of patients. In an immunohistochemical study of 3 patients, Stoppacciaro et al11 have shown that a complex network of cytokines and chemokines regulates histiocyte recruitment and accumulation in the lesions. More recently, Dagna et al12 assessed spontaneous and stimulated cytokine production by mononuclear cells obtained from biopsy fragments in a single patient. This study revealed production of tumor necrosis factor-α (TNF-α) after stimulation, and spontaneous secretion of IL-6 and CXC chemokine ligand 8/IL-8 (CXCL8/IL-8), this latter being known as a chemoattractant for polymorphonuclear cells and monocytes. Finally, Aouba et al7 have provided some evidence in 2 patients that targeting the IL-1 pathway might be an appro-priate strategy.
Although the data we described have undoubtedly contributed to our understanding of the immune responses in ECD, the lack of a systematic large-scale assessment of major cytokine blood levels has prevented us from further identifying the systemic immunologic modulators associated with the disease. In this study, we quantitatively analyzed 23 cytokines, chemokines, and growth factors in serum samples obtained from a single-center cohort of 37 patients with ECD. This work represents a unique opportunity to identify systemic immune perturbations and new targets in a disease for which therapeutic strategy is not well established.
Methods
Patients and controls
Serum samples were obtained from 37 patients with ECD (27 male and 10 female; median age, 61 years [range, 21-80 years]) followed in the Department of Internal Medicine, Pitié-Salpêtrière Hospital, Paris, France, between January 2001 and June 2010, and in an identical number of healthy subjects (median age, 57 years [range, 20-77 years]). In all these patients, ECD was diagnosed based on (1) typical histologic findings, such as, infiltration with foamy histiocytes nested among polymorphic granuloma and fibrosis or xanthogranulomatosis with CD68+ and CD1a− immunohistochemical staining, which is typical of histiocytes in ECD; and (2) typical skeletal findings with x-ray scans showing bilateral and symmetrical cortical osteosclerosis of the diaphyseal and metaphyseal regions in the long bones and/or symmetrical and abnormally strong labeling of the distal ends of the long bones shown on Technetium-99 bone scintigraphy. These criteria for ECD were used in our previous studies.2,6,13 Median disease duration was 24 months (range, 0-335 months). There were 15 patients free of any treatment and 22 patients who received IFN-α or pegylated IFN-α at various doses, for a median duration of 24.2 months (range, 1.9-175.6 months). Detailed patient characteristics and treatments are presented in Table 1. Informed consent and approval by the Hôpital Pitié-Salpêtrière Institutional Review Board were obtained before the study began in accordance with the Declaration of Helsinki.
Characteristics and treatments of the 37 patients with ECD
| Patient no. . | Sex/age, y . | Disease duration, mo . | Topography of lesions . | Therapy . | Duration of IFN treatment, mo . |
|---|---|---|---|---|---|
| 1 | M/39 | 30.0 | B, MS, LV, H, S, O, CNS, P, L, R | None | ND |
| 2 | M/49 | 0.0 | B, MS, P, R | None | ND |
| 3 | F/73 | 0.0 | B | None | ND |
| 4 | F/59 | 19.1 | B, MS, LV, H, L, R | None | ND |
| 5 | M/47 | 47.7 | B, MS, H, S, O, CNS | None | ND |
| 6 | F/79 | 79.7 | B, LV | None | ND |
| 7 | M/70 | 0.0 | B, MS, LV, H, O, R | None | ND |
| 8 | M/49 | 0.5 | B, LV, H, R | None | ND |
| 9 | M/53 | 0.0 | B, S, P, R | None | ND |
| 10 | M/70 | 0.0 | MS, LV, H, CNS | None | ND |
| 11 | M/45 | 40.3 | B, MS, LV, CNS, L | None | ND |
| 12 | M/80 | 1.8 | B, H, R | None | ND |
| 13 | F/42 | 0.0 | B, LV, H, L | None | ND |
| 14 | M/69 | 0.5 | B, LV, H, S, O, CNS, R | None | ND |
| 15 | M/44 | 6.5 | B, S, L, R | None | ND |
| 16 | M/60 | 4.9 | B, MS, LV, H, S, CNS, L, R | IFN-6 MIU 3×/wk | 4.8 |
| 17 | M/71 | 335.4 | B, MS, LV, CNS, P, L | IFN-3 MIU 3×/wk | 48.8 |
| 18 | F/62 | 28.3 | B, MS, H, CNS, L | IFN-9 MIU 3×/wk | 28.3 |
| 19 | M/61 | 85.7 | B, R | Pegylated IFN 135 μg/wk | 84.7 |
| 20 | M/80 | 24.4 | B, MS, H, L | Pegylated IFN 180 μg/wk | 16.8 |
| 21 | M/56 | 9.5 | B, MS, LV, H, R | Prednisone 12 mg/d + IFN-3 MIU 3×/wk | 8.9 |
| 22 | M/31 | 61.4 | B, MS, CNS, P | IFN-9 MIU 3×/wk | 12.3 |
| 23 | M/63 | 121.5 | B, LV, H, S, CNS, L, R | Pegylated IFN 180 μg/wk | 51.9 |
| 24 | M/56 | 5.3 | B, MS, P, R | IFN-3 MIU 3×/wk | 1.9 |
| 25 | M/74 | 36.9 | B, LV, H, L, R | Pegylated IFN 135 μg/wk | 28.6 |
| 26 | M/66 | 56.7 | B, MS, LV, H, S, O, CNS, L | IFN-9 MIU 3×/wk | 32.9 |
| 27 | M/63 | 12.0 | B, MS, LV, CNS, R | IFN-6 MIU 3×/wk | 7.1 |
| 28 | F/70 | 7.9 | B, LV, H, O, R | IFN-3 MIU 3×/wk | 6.0 |
| 29 | M/63 | 88.6 | B, LV, H, CNS, P, L | IFN-9 MIU 3×/wk | 20.6 |
| 30 | M/67 | 21.2 | B, MS, L | Pegylated IFN 135 μg/wk | 13.8 |
| 31 | M/61 | 37.0 | B, MS, LV, CNS, L, R | Pegylated IFN 90 μg/wk | 33.9 |
| 32 | F/56 | 44.2 | B | Pegylated IFN 180 μg/wk | 24.3 |
| 33 | F/21 | 53.1 | MS, P, L, R | Pegylated IFN 135 μg/wk | 24.2 |
| 34 | M/55 | 18.5 | B, MS, LV, H, S, O, CNS, L, R | IFN-9 MIU 3×/wk | 17.0 |
| 35 | M/52 | 176.0 | B, MS, S, O, R | IFN-6 MIU 3×/wk | 175.6 |
| 36 | F/66 | 38.4 | B, LV, H | Pegylated IFN 180 μg/wk | 31.2 |
| 37 | F/76 | 30.6 | B, MS, LV, H, R | Pegylated IFN 180 μg/wk | 28.3 |
| Patient no. . | Sex/age, y . | Disease duration, mo . | Topography of lesions . | Therapy . | Duration of IFN treatment, mo . |
|---|---|---|---|---|---|
| 1 | M/39 | 30.0 | B, MS, LV, H, S, O, CNS, P, L, R | None | ND |
| 2 | M/49 | 0.0 | B, MS, P, R | None | ND |
| 3 | F/73 | 0.0 | B | None | ND |
| 4 | F/59 | 19.1 | B, MS, LV, H, L, R | None | ND |
| 5 | M/47 | 47.7 | B, MS, H, S, O, CNS | None | ND |
| 6 | F/79 | 79.7 | B, LV | None | ND |
| 7 | M/70 | 0.0 | B, MS, LV, H, O, R | None | ND |
| 8 | M/49 | 0.5 | B, LV, H, R | None | ND |
| 9 | M/53 | 0.0 | B, S, P, R | None | ND |
| 10 | M/70 | 0.0 | MS, LV, H, CNS | None | ND |
| 11 | M/45 | 40.3 | B, MS, LV, CNS, L | None | ND |
| 12 | M/80 | 1.8 | B, H, R | None | ND |
| 13 | F/42 | 0.0 | B, LV, H, L | None | ND |
| 14 | M/69 | 0.5 | B, LV, H, S, O, CNS, R | None | ND |
| 15 | M/44 | 6.5 | B, S, L, R | None | ND |
| 16 | M/60 | 4.9 | B, MS, LV, H, S, CNS, L, R | IFN-6 MIU 3×/wk | 4.8 |
| 17 | M/71 | 335.4 | B, MS, LV, CNS, P, L | IFN-3 MIU 3×/wk | 48.8 |
| 18 | F/62 | 28.3 | B, MS, H, CNS, L | IFN-9 MIU 3×/wk | 28.3 |
| 19 | M/61 | 85.7 | B, R | Pegylated IFN 135 μg/wk | 84.7 |
| 20 | M/80 | 24.4 | B, MS, H, L | Pegylated IFN 180 μg/wk | 16.8 |
| 21 | M/56 | 9.5 | B, MS, LV, H, R | Prednisone 12 mg/d + IFN-3 MIU 3×/wk | 8.9 |
| 22 | M/31 | 61.4 | B, MS, CNS, P | IFN-9 MIU 3×/wk | 12.3 |
| 23 | M/63 | 121.5 | B, LV, H, S, CNS, L, R | Pegylated IFN 180 μg/wk | 51.9 |
| 24 | M/56 | 5.3 | B, MS, P, R | IFN-3 MIU 3×/wk | 1.9 |
| 25 | M/74 | 36.9 | B, LV, H, L, R | Pegylated IFN 135 μg/wk | 28.6 |
| 26 | M/66 | 56.7 | B, MS, LV, H, S, O, CNS, L | IFN-9 MIU 3×/wk | 32.9 |
| 27 | M/63 | 12.0 | B, MS, LV, CNS, R | IFN-6 MIU 3×/wk | 7.1 |
| 28 | F/70 | 7.9 | B, LV, H, O, R | IFN-3 MIU 3×/wk | 6.0 |
| 29 | M/63 | 88.6 | B, LV, H, CNS, P, L | IFN-9 MIU 3×/wk | 20.6 |
| 30 | M/67 | 21.2 | B, MS, L | Pegylated IFN 135 μg/wk | 13.8 |
| 31 | M/61 | 37.0 | B, MS, LV, CNS, L, R | Pegylated IFN 90 μg/wk | 33.9 |
| 32 | F/56 | 44.2 | B | Pegylated IFN 180 μg/wk | 24.3 |
| 33 | F/21 | 53.1 | MS, P, L, R | Pegylated IFN 135 μg/wk | 24.2 |
| 34 | M/55 | 18.5 | B, MS, LV, H, S, O, CNS, L, R | IFN-9 MIU 3×/wk | 17.0 |
| 35 | M/52 | 176.0 | B, MS, S, O, R | IFN-6 MIU 3×/wk | 175.6 |
| 36 | F/66 | 38.4 | B, LV, H | Pegylated IFN 180 μg/wk | 31.2 |
| 37 | F/76 | 30.6 | B, MS, LV, H, R | Pegylated IFN 180 μg/wk | 28.3 |
Data for age is at serum sampling; disease duration is from histologic diagnosis of ECD. Therapy is at the time of serum sampling.
B indicates long bones; MS, maxillary sinus; LV, large vessels; H, heart; S, skin, O, orbit; CNS, central nervous system; P, pituitary gland; L, lungs; R, retroperitoneal; MIU, million international units; and ND, none determined.
Sample preparation and cytokine measurement
Venous blood samples were collected into BD Vacutainer tubes containing clot activator, and processed immediately. After centrifugation at 2000g for 10 minutes, serum samples were stored at −80°C and thawed once only. Clots of fibrin were removed from defrosted samples by a second centrifugation at 12 000g for 10 minutes, and these samples were immediately used for cytokine measurement using Invitrogen Luminex Human Cytokine 25-plex antibody bead kit. All samples were masked for subject identity and analyzed according to the manufacturer's instructions at a sample dilution of 1/4.
Immunohistochemisty
For immunohistochemical analysis, 4-μm-thick sections were cut from paraffin-embedded blocks obtained in 6 untreated patients. Samples were obtained from blocks containing infiltrated pericardium (patient no. 4), perirenal fat (patients no. 6, 7, and 9), peritoneum (patient no. 14), and aorta (patient no. 15). Sections were deparaffinized then rehydrated and heated in citrate buffer (pH 6.0) for 30 minutes at 97°C. Endogenous peroxidase activity was blocked using peroxidase block and sequentially incubated with mouse anti–human CD123 antibody (BD Pharmingen) for 1 hour, and visualized with the biotin-free polymeric visualization system Ultravison LP (Lab Vision). Sections were then stained with the chromogen diaminobenzidine for 5 minutes, and counterstained with hematoxylin. A sample from a patient with a case of cutaneous blastic plasmacytoid dendritic cell (pDC) tumor was used as positive control.
Statistical analysis
IL-2RA and RANTES were excluded from analysis because of inadequate bead counts and unreliable performance of standard curve, respectively. Samples with nondetectable values were replaced with zero for the purpose of continuous data analyses. Quantitative data were expressed as median (minimum-maximum) values. The nonparametric Mann-Whitney U test was used for comparison of continuous data between groups. The Wilcoxon signed-rank test was used for comparison of cytokine levels among patients with available follow-up serum samples. Correlation between age at compilation, disease duration, and cytokine levels was calculated using Spearman rank-order test. Association between cytokine levels and presence of any type of visceral involvement (as reported in Table 1) was assessed using logistic regression. All these analyses were followed by Bonferroni type I error rate correction, when needed. Exploratory principal component analysis was used to assess graphically the separation between patients and controls, with regard to the cytokine profile that was identified. Statistical significance was defined as P < .05 or P < .05/23 (P < .002) when Bonferroni correction was applied. Statistical analyses were performed using Prism software Version 5.0 (GraphPad) and JMP8 (SAS Institute).
Results
Cytokine profiles
We found no correlation between age at sampling or disease duration and cytokine levels, as well as no statistical association between cytokine levels and presence of any type of visceral involvement (data not shown).
Comparisons of median serum levels of cytokines between untreated patients with ECD and controls are presented in Table 2 and Figure 1. A total of 4 of the 23 cytokines analyzed had significantly higher levels among untreated patients: IL-6 displayed a 16-fold increase (P = .0002), IFN-α a 5.3-fold increase (P < .0001), IL-12 a 2.3-fold increase (P < .0001), and monocyte chemotactic protein-1 (MCP-1) a 2-fold increase (P < .0001). Conversely, IL-7 and IL-4, respectively, displayed a 130- and 3.5-fold increase in controls, compared with patients (P < .0001 in both).
Serum concentrations of cytokines in untreated and IFN-α–treated patients with ECD and controls
| Cytokine . | Cytokine concentration, pg/mL* . | P . | |||
|---|---|---|---|---|---|
| Untreated patients (n = 15) . | IFN-treated patients (n = 22) . | Healthy controls (n = 37) . | Untreated vs controls . | IFN-treated vs controls . | |
| TNF-α | 0.0 (0.0-68.0) | 0.0 (0.0-55.6) | 0.0 (0.0-15.6) | .03 | .11 |
| IL-1β | 0.0 (0.0-59.2) | 0.0 (0.0-819.6) | 12.1 (0.0-46.6) | .08 | .01 |
| IL-1RA | 326.3 (158.0-3259.1) | 405.7 (158.0-1918.6) | 189.8 (82.1-275.4) | .01 | < .0001† |
| IL-2 | 0.0 (0.0-0.0) | 0.0 (0.0-130.3) | 0.0 (0.0-0.0) | > .999 | .19 |
| IL-4 | 9.6 (0.0-57.8) | 9.6 (0.0-291.1) | 33.6 (22.0-53.0) | < .0001† | < .0001† |
| IL-5 | 0.0 (0.0-6.1) | 0.0 (0.0-369.6) | 0.0 (0.0-7.4) | .59 | .64 |
| IL-6 | 16.1 (0.0-326.7) | 0.0 (0.0-1394.8) | 0.0 (0.0-19.0) | .0002† | .22 |
| IL-7 | 0.0 (0.0-113.6) | 0.0 (0.0-751.7) | 130.2 (94.4-290.6) | < .0001† | < .0001† |
| IL-8 | 29.0 (0.0-898.4) | 30.3 (0.0-2342.6) | 39.8 (0.0-333.6) | .72 | .95 |
| IL-10 | 0.0 (0.0-0.0) | 0.0 (0.0-106.0) | 0.0 (0.0-0.0) | > .999 | .20 |
| IL-12 | 179.4 (134.8-986.8) | 192.1 (90.2-457.6) | 76.2 (44.0-118.8) | < .0001† | < .0001† |
| IL-13 | 36.0 (0.06-144.2) | 36.0 (0.0-714.0) | 21.0 (0.0-58.3) | .05 | < .0001† |
| IL-15 | 0.0 (0.0-78.4) | 0.0 (0.0-391.4) | 0.0 (0.0-0.0) | .02 | .02 |
| IL-17 | 33.0 (0.0-348.8) | 9.2 (0.0-210.6) | 22.8 (0.0-71.3) | .08 | .91 |
| IFN-α | 111.0 (82.8-331.2) | 239.8 (60.0-2065.8) | 20.9 (13.4-41.0) | < .0001† | < .0001† |
| IFN-γ | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-20.3) | .26 | .17 |
| IP-10 | 25.4 (0.0-342.2) | 38.3 (11.5-261.0) | 10.6 (0.0-49.2) | .02 | < .0001† |
| MCP-1 | 460.3 (200.1-2657.0) | 492.4 (104.9-3405.2) | 220.6 (106.5-376.5) | < .0001† | < .0001† |
| MIG | 52.2 (0.0-883.7) | 52.2 (0.0-141.8) | 49.9 (28.6-67.5) | .14 | .76 |
| MIP-1α | 61.3 (47.8-274.7) | 58.7 (0.0-440.6) | 83.6 (58.8-98.7) | .02 | .0009† |
| MIP-1β | 30.0 (0.0-132.8) | 32.1 (0.0-546.0) | 30.4 (0.0-76.9) | .94 | .66 |
| Eotaxin | 62.8 (0.0-220.8) | 53.1 (20.3-646.9) | 95.6 (26.0-219.2) | .03 | .003 |
| GM-CSF | 23.0 (0.0-208.2) | 0.0 (0.0-341.0) | 0.0 (0.0-57.6) | .10 | .35 |
| Cytokine . | Cytokine concentration, pg/mL* . | P . | |||
|---|---|---|---|---|---|
| Untreated patients (n = 15) . | IFN-treated patients (n = 22) . | Healthy controls (n = 37) . | Untreated vs controls . | IFN-treated vs controls . | |
| TNF-α | 0.0 (0.0-68.0) | 0.0 (0.0-55.6) | 0.0 (0.0-15.6) | .03 | .11 |
| IL-1β | 0.0 (0.0-59.2) | 0.0 (0.0-819.6) | 12.1 (0.0-46.6) | .08 | .01 |
| IL-1RA | 326.3 (158.0-3259.1) | 405.7 (158.0-1918.6) | 189.8 (82.1-275.4) | .01 | < .0001† |
| IL-2 | 0.0 (0.0-0.0) | 0.0 (0.0-130.3) | 0.0 (0.0-0.0) | > .999 | .19 |
| IL-4 | 9.6 (0.0-57.8) | 9.6 (0.0-291.1) | 33.6 (22.0-53.0) | < .0001† | < .0001† |
| IL-5 | 0.0 (0.0-6.1) | 0.0 (0.0-369.6) | 0.0 (0.0-7.4) | .59 | .64 |
| IL-6 | 16.1 (0.0-326.7) | 0.0 (0.0-1394.8) | 0.0 (0.0-19.0) | .0002† | .22 |
| IL-7 | 0.0 (0.0-113.6) | 0.0 (0.0-751.7) | 130.2 (94.4-290.6) | < .0001† | < .0001† |
| IL-8 | 29.0 (0.0-898.4) | 30.3 (0.0-2342.6) | 39.8 (0.0-333.6) | .72 | .95 |
| IL-10 | 0.0 (0.0-0.0) | 0.0 (0.0-106.0) | 0.0 (0.0-0.0) | > .999 | .20 |
| IL-12 | 179.4 (134.8-986.8) | 192.1 (90.2-457.6) | 76.2 (44.0-118.8) | < .0001† | < .0001† |
| IL-13 | 36.0 (0.06-144.2) | 36.0 (0.0-714.0) | 21.0 (0.0-58.3) | .05 | < .0001† |
| IL-15 | 0.0 (0.0-78.4) | 0.0 (0.0-391.4) | 0.0 (0.0-0.0) | .02 | .02 |
| IL-17 | 33.0 (0.0-348.8) | 9.2 (0.0-210.6) | 22.8 (0.0-71.3) | .08 | .91 |
| IFN-α | 111.0 (82.8-331.2) | 239.8 (60.0-2065.8) | 20.9 (13.4-41.0) | < .0001† | < .0001† |
| IFN-γ | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-20.3) | .26 | .17 |
| IP-10 | 25.4 (0.0-342.2) | 38.3 (11.5-261.0) | 10.6 (0.0-49.2) | .02 | < .0001† |
| MCP-1 | 460.3 (200.1-2657.0) | 492.4 (104.9-3405.2) | 220.6 (106.5-376.5) | < .0001† | < .0001† |
| MIG | 52.2 (0.0-883.7) | 52.2 (0.0-141.8) | 49.9 (28.6-67.5) | .14 | .76 |
| MIP-1α | 61.3 (47.8-274.7) | 58.7 (0.0-440.6) | 83.6 (58.8-98.7) | .02 | .0009† |
| MIP-1β | 30.0 (0.0-132.8) | 32.1 (0.0-546.0) | 30.4 (0.0-76.9) | .94 | .66 |
| Eotaxin | 62.8 (0.0-220.8) | 53.1 (20.3-646.9) | 95.6 (26.0-219.2) | .03 | .003 |
| GM-CSF | 23.0 (0.0-208.2) | 0.0 (0.0-341.0) | 0.0 (0.0-57.6) | .10 | .35 |
MIP indicates macrophage-inflammatory protein; MIG, monokine induced by IFN-γ; and GM-CSF, granulocyte-macrophage colony-stimulating factor.
Expressed in median (minimum-maximum). Samples with nondetectable cytokine levels were considered to be 0.0 pg/mL.
Values remain significant after Bonferroni correction for multiple testing.
Comparison of serum cytokine levels. Comparison of IFN-α, IP-10, IL-1RA, IL-12, IL-6, MCP-1, IL-13, IL-4, IL-7, and MIP-1α between untreated patients with ECD (n = 15), IFN-α–treated patients with ECD (n = 22), and controls (n = 37). Untreated indicates untreated patients; Treated, IFN-α–treated patients; NS, nonsignificant P value.
Comparison of serum cytokine levels. Comparison of IFN-α, IP-10, IL-1RA, IL-12, IL-6, MCP-1, IL-13, IL-4, IL-7, and MIP-1α between untreated patients with ECD (n = 15), IFN-α–treated patients with ECD (n = 22), and controls (n = 37). Untreated indicates untreated patients; Treated, IFN-α–treated patients; NS, nonsignificant P value.
Comparisons of cytokine levels between IFN-α–treated patients and controls are presented in Table 2 and Figure 1. Of the 23 cytokines analyzed, 6 showed significantly higher levels among patients treated with IFN-α: IFN-α displayed a 11.5-fold increase (P < .0001), IFN-γ–inducible protein 10 (IP-10) a 3.6-fold increase (P < .0001), IL-12 a 2.5-fold increase (P < .0001), MCP-1 a 2.2-fold increase (P < .0001), IL-1RA a 2.1-fold increase (P < .0001), and IL-13 a 1.7-fold increase (P < .0001). Conversely, IL-7, IL-4, and macrophage inflammatory protein 1α (MIP-1α), respectively, displayed a 130-fold increase (P < .0001), a 3.5-fold increase (P < .0001), and a 1.4-fold increase (P = .0009) in controls, compared with patients treated with IFN-α.
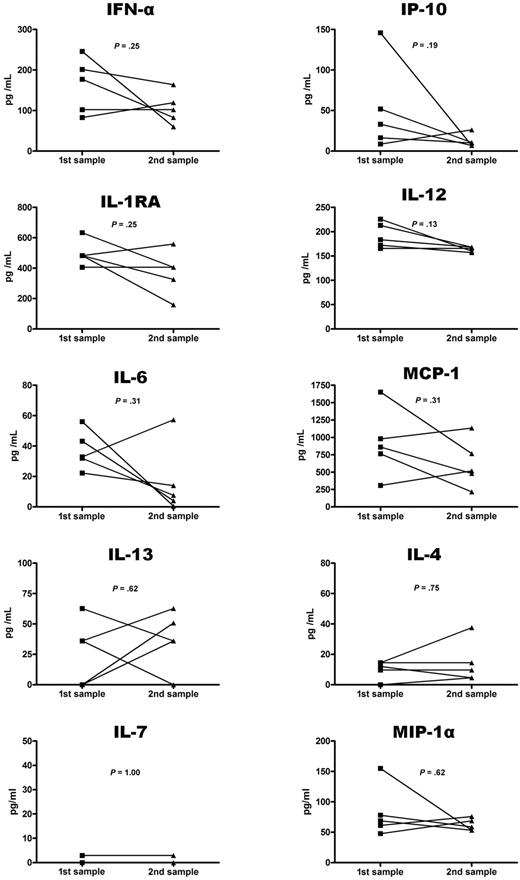
Interestingly, cytokine profiles proved to be particularly stable at the individual level. In particular, 5 untreated patients (patients no. 4, 6, 13, 14, and 15) had available follow-up serum samples (median interval during the initial and the follow-up sample: 8 months [4-105 months]). None of the 23 cytokines analyzed displayed any significant change between the initial and follow-up evaluation (Figure 2). Similarly, comparison of cytokine levels between the 15 untreated patients and the 22 IFN-α–treated patients yielded no significant difference (Figure 1). Principal component analysis further confirmed that controls and patients could be distinguished based on their production of IFN-α, IL-12, MCP-1, IL-4, and IL-7 (Figure 3). Of note, untreated and IFN-α–treated patients could not be distinguished based on this cytokine profile, suggesting this profile is not modified by IFN-α treatment.
Comparison of initial and follow-up serum levels in patients with ECD. Comparison of initial and follow-up serum levels of 10 cytokines in 5 patients with ECD.
Comparison of initial and follow-up serum levels in patients with ECD. Comparison of initial and follow-up serum levels of 10 cytokines in 5 patients with ECD.
Component analysis of cytokine levels. Principal component analysis of IFN-α, IL-12, MCP-1, IL-4, and IL-7 production in patients with ECD and healthy controls. Analysis confirms that patients and controls may be distinguished based on a 5-cytokine profile (IFN-α, IL-12, MCP-1, IL-4, and IL-7), as they form 2 separate groups, but that treated and untreated patients are undistinguishable. There were 4 patients projected out of the analysis and thus were not represented for clarity reasons.
Component analysis of cytokine levels. Principal component analysis of IFN-α, IL-12, MCP-1, IL-4, and IL-7 production in patients with ECD and healthy controls. Analysis confirms that patients and controls may be distinguished based on a 5-cytokine profile (IFN-α, IL-12, MCP-1, IL-4, and IL-7), as they form 2 separate groups, but that treated and untreated patients are undistinguishable. There were 4 patients projected out of the analysis and thus were not represented for clarity reasons.
Anti–CD123 immunohistochemisty
It is already known that several cytokines we detected systematically are produced in ECD lesions.11 It remains unknown, however, whether IFN-α is also produced locally. The main source of this cytokine is the CD68+CD1a−CD123+ pDC subset. Presence of pDC in the lesions was therefore investigated in 6 patients with ECD, using an anti–CD123 antibody. Although the positive control (a cutaneous blastic pDC tumor sample) was clearly stained by the anti–CD123 antibody, 5 of 6 ECD samples showed no such staining (Figure 4), and a single sample (no. 4) showed only minor staining. This result emphasizes that the tissular infiltrate of ECD does not comprise a significant proportion of pDCs, and thus that this infiltrate is unlikely the primary source of IFN-α production in ECD.
CD123 staining in patients with ECD. ECD anti–CD123 immunostaining is negative in 5 cases (patients no. 6, 7, 9, 14, and 15); in 1 case (patient no. 4) rare cells are positive for CD123 (filled arrowheads). Samples were obtained from blocks containing infiltrated pericardium (patient no. 4), perirenal fat (patients no. 6, 7, and 9), peritoneum (patient no. 14), and aorta (patient no. 15). A cutaneous blastic pDC tumor sample was used as positive control (immunoperoxidase method, magnification ×200).
CD123 staining in patients with ECD. ECD anti–CD123 immunostaining is negative in 5 cases (patients no. 6, 7, 9, 14, and 15); in 1 case (patient no. 4) rare cells are positive for CD123 (filled arrowheads). Samples were obtained from blocks containing infiltrated pericardium (patient no. 4), perirenal fat (patients no. 6, 7, and 9), peritoneum (patient no. 14), and aorta (patient no. 15). A cutaneous blastic pDC tumor sample was used as positive control (immunoperoxidase method, magnification ×200).
Discussion
In this study, we quantitatively analyzed 23 different cytokines, chemokines, and growth factors in serum samples obtained from 37 patients with ECD, including 15 untreated and 22 IFN-α–treated patients. Our data represent the largest scale for an immunologic evaluation of ECD patients to date.
Previous pathologic studies revealed that the cellular infiltrate of ECD comprised a significant contingent of T-cell helper 1 (Th-1) lymphocytes, as indicated by their prominent IFN-γ staining.11 Moreover, infiltrating histiocytes were found to express CXCL10/IP-10, which is an IFN-γ–induced chemokine.11 Although we observed no significant difference in the serum levels of IFN-γ, we found a significant increase of IL-12, a cytokine known to favor the Th-1 pathway,14 and a significant decrease of IL-4, a major T-cell helper 2 cytokine,15 in both treated and untreated patients. We also observed a significant increase in the serum levels of IP-10 in patients treated with IFN-α. Altogether, these data strongly suggests that a Th-1–mediated systemic immune response is taking place in ECD.
Proinflammatory cytokines induced by Th-1 responses, such as IL-1, TNF-α, and IL-6, are strongly expressed in ECD lesions.11 Although increased serum levels of IL-1 and TNF-α have been previously reported in a limited number of patients,5,7 we observed no such increase in this cohort of 37 patients with ECD. Interestingly, a dissociation between IL-1 and IL-1RA has been reported during the resolution of collagen-induced arthritis,16 and we found significantly raised levels of IL-1RA in patients treated with IFN-α. Moreover, we found a significant increase of IL-6 in untreated patients, but not in IFN-α–treated patients. Altogether these data suggest the IL-1/IL-1RA and IL-6 pathways play a key role in ECD. Interestingly, IL-6 as well as IL-7, a pleiotropic cytokine involved in differentiation and homeostasis of B cells and T cells17,18 we found decreased in ECD patients, have been involved in osteoclast differentiation and bone resorption.19,20 This finding may provide a link with the typical osteosclerosis of the long bones encountered among patients with ECD.
Inflammatory chemokines play a pivotal role in the control of histiocyte and lymphocyte recruitment.21 Stoppacciaro et al11 have shown that CC chemokine ligand (CCL) in CCL2/MCP-1, CCL4/MIP-1β, and CCL5/regulated on activation normal T cell expressed and secreted were strongly expressed in ECD lesions. In this study, we observed a significant increase in the serum level of CCL2/MCP-1 in both treated and untreated patients, whereas levels of CCL3/MIP-1α were significantly decreased in IFN-α–treated patients, suggesting a key role of these chemokines for systemic recruitment of histiocytes.
Interestingly, we found a significant increase in IFN-α serum levels in untreated patients, compared with controls. Type I IFN production is initiated at the early stages of the innate immune response.22 IFN-α is thus believed to be one of the dominant factors shaping downstream events in the innate and adaptive immune responses, such as favoring Th-1 differentiation23 and enhancing proinflammatory cytokine signaling,24 which is consistent with the other findings observed in this study. Although the most potent source of IFN-α are the pDCs,25 in situ immunohistochemical studies suggested that the infiltrate does not contain pDCs, and thus that ECD lesions are likely not the main source of IFN-α production. The exact origin of IFN-α production thus remains to be determined in ECD patients.
Paradoxically, increased levels of IFN-α have been reported in several inflammatory diseases,26-29 and IFN-α was found effective for treating some of these diseases, including chronic hepatitis C virus infection and cutaneous melanoma.30,31 Similarly, we found increased levels of IFN-α in untreated patients, and treatment with recombinant IFN-α has been shown to be an effective therapeutic option in ECD.6,32 In this study, we found no significant difference between cytokine levels measured in untreated and IFN-α–treated patients. This suggests that IFN-α has limited impact on the systemic immunologic perturbations that are characteristic of ECD. We also used biomathematical modelization to demonstrate that a 5-cytokine signature (IFN-α, IL-12, MCP-1, IL-4, and IL-7) could distinguish patients from controls, but not untreated patients from IFN-α–treated patients, suggesting that patients with ECD treated with IFN-α only have a limited immunologic response. Interestingly, such an analytical strategy could be used to further monitor therapeutic efficacy in ECD. In principle, one could expect to observe similar perturbations of the cytokine network in response to various clinical conditions ultimately leading to systemic inflammation. However, the cytokine signature described in this study appeared truly unique to ECD, as a similar profile was not identified in other inflammatory diseases such as rheumatoid arthritis,33 systemic sclerosis,34 idiopathic inflammatory myopathies,35 relapsing polychondritis,36 or sepsis.37,38 Therefore, this approach appears to be multiparametric enough to allow the description of disease-specific signatures. This relationship is probably because the common pathways are implicated in the pathogenesis of various diseases, but at various degrees.
In conclusion, our data reveal an intense systemic immune activation mainly involving IFN-α, IL-1/IL1-RA, IL-6, IL-12, and MCP-1. The present study further underlines the systemic immune Th-1–oriented perturbation associated with this condition, and provides clues for the choice of more focused therapeutic agents.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Annette Lesot for kind technical support.
Authorship
Contribution: L.A. designed and organized research, performed experiments, performed statistical analysis, analyzed data, designed the figures, and wrote the paper; G.G., Z.A., and J.H. designed research, analyzed data, and wrote the paper; F.C. performed anti–CD123 immunohistochemical analysis, analyzed data, and edited the manuscript; V.L., C.P., and P.G.-D. performed experiments and edited the manuscript; M.L., B.H., J.-E.K., and L.M., analyzed data and edited the manuscript; and C.D. provided serum samples and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julien Haroche, MD, PhD, Service de Médecine Interne 2, Groupe Hospitalier Pitié-Salpêtrière, 47-83, blvd de l'Hôpital, 75013 Paris, France; e-mail: julien.haroche@psl.aphp.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal