Key Points
At 3 years, A+AVD showed a robust benefit vs ABVD that is independent of PET2 status.
Benefit of A+AVD vs ABVD was observed in prespecified subgroups and was independent of disease stage, age, and prognostic risk score.
Abstract
The phase 3 ECHELON-1 study demonstrated that brentuximab vedotin (A) with doxorubicin, vinblastine, and dacarbazine (AVD; A+AVD) exhibited superior modified progression-free survival (PFS) vs doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for frontline treatment of patients with stage III/IV classical Hodgkin lymphoma (cHL). Maturing positron emission tomography (PET)-adapted trial data highlight potential limitations of PET-adapted approaches, including toxicities with dose intensification and higher-than-expected relapse rates in PET scan after cycle 2 (PET2)-negative (PET2−) patients. We present an update of the ECHELON-1 study, including an exploratory analysis of 3-year PFS per investigator. A total of 1334 patients with stage III or IV cHL were randomized 1:1 to receive 6 cycles of A+AVD (n = 664) or ABVD (n = 670). Interim PET2 was required. At median follow-up of 37 months, 3-year PFS rates were 83.1% with A+AVD and 76.0% with ABVD; 3-year PFS rates in PET2− patients aged <60 years were 87.2% vs 81.0%, respectively. A beneficial trend in PET2+ patients aged <60 years on A+AVD was also observed, with a 3-year PFS rate of 69.2% vs 54.7% with ABVD. The benefit of A+AVD in the intent-to-treat population appeared independent of disease stage and prognostic risk factors. Upon continued follow-up, 78% of patients with peripheral neuropathy on A+AVD had either complete resolution or improvement compared with 83% on ABVD. These data highlight that A+AVD provides a durable efficacy benefit compared with ABVD for frontline stage III/IV cHL, consistent across key subgroups regardless of patient status at PET2, without need for treatment intensification or bleomycin exposure. This trial was registered at www.clinicaltrials.gov as #NCT01712490 (EudraCT no. 2011-005450-60).
Introduction
The combination of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) was first described over 40 years ago for treatment of patients with newly diagnosed advanced Hodgkin lymphoma (HL), and, in 1992, demonstrated superiority vs the then standard-of-care combination of mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) in a randomized phase 3 study.1 However, ∼24% to 39% of patients with advanced HL are refractory to or relapse following frontline treatment with ABVD.2-4
In an effort to improve patient outcomes and minimize the risk of short- and long-term toxicities for patients with newly diagnosed advanced HL, several contemporary clinical trials have assessed ABVD-based positron emission tomography (PET)-adapted strategies, including the RATHL, GITIL/FIL HD 0607, and SWOG S0816 studies.5-8 Data from the RATHL study of ABVD with escalation to bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) or de-escalation to doxorubicin, vinblastine, and dacarbazine (AVD) in patients with stage IIB, III, or IV HL found that PET-adapted de-escalation to AVD failed to demonstrate noninferiority compared with ABVD, although a lower incidence of pulmonary toxic effects was observed following the omission of bleomycin from the regimen after cycle 2.5 Additional adaptations attempting to further improve outcomes have also been explored, such as the addition of rituximab to BEACOPP for interim PET scan after cycle 2 (PET2)-positive (PET2+) patients and evaluation of the role of consolidative radiotherapy in PET2-negative (PET2−) patients with a large nodal mass (≥5 cm) at diagnosis in the GITIL/FIL HD 0607 trial.6 The authors concluded that the addition of rituximab to BEACOPP following ABVD did not confer an additional efficacy benefit. Patients with initial bulky nodal sites of involvement who achieved interim and posttreatment negative PET scans were randomized to consolidative radiotherapy or no further treatment. The addition of consolidative radiotherapy for these patients did not confer a progression-free survival (PFS) benefit.6 At 3 years, no secondary malignancies were reported.
However, a recent 5-year follow-up from the SWOG S0816 trial demonstrated some potential limitations of the PET-adapted treatment approach.8 Although escalation of therapy from ABVD to BEACOPP in PET2+ patients improved 2-year PFS compared with historical estimates of 30% to 64%, longer-term follow-up found a notable increase in the rate of secondary malignancies.7-9 Additionally, with a longer follow-up a higher-than-expected rate of relapse was noted in PET2− patients treated with 6 cycles of ABVD.7,8 As patients with HL are likely to have a longer life expectancy, and with emerging data suggesting increased rates of short- and long-term toxicities (eg, secondary malignancies, infertility) in patients receiving escalated therapy and higher-than-expected rates of relapse in PET2− patients,5 it is clear that additional treatment options are needed. Additionally, the response-adapted treatment approach requires real-time availability and standardized interpretation of PET scans.
ECHELON-1 is a large, international, open-label, randomized, multicenter, phase 3 trial comparing brentuximab vedotin (A) with AVD (A+AVD) vs ABVD as frontline therapy for patients with stage III or IV classical HL (cHL). ECHELON-1’s primary end point is modified PFS per independent review facility, defined as disease progression, death, or the receipt of additional treatment by patients not achieving complete response, as reviewed by an independent committee, at the end of frontline therapy.10 At the primary analysis, with a median follow-up of 24.6 months, ECHELON-1 demonstrated that 6 cycles of A+AVD was superior to ABVD, with 2-year modified PFS rates of 82.1% and 77.2% (hazard ratio [HR] = 0.77; P = .04), respectively.10 Overall, compared with ABVD, treatment with A+AVD was associated with higher rates of febrile neutropenia (19% vs 8%), which were decreased through the use of primary prophylaxis with granulocyte colony-stimulating factor (G-CSF), increased rates of peripheral neuropathy (PN; 67% vs 43%), and lower rates of pulmonary-related toxicity (2% vs 7%).10
Here, we present a 3-year update of ECHELON-1, including PFS per investigator in the intent-to-treat (ITT) population, results by PET2 status, age, prognostic risk scores, and PN incidence and resolution.
Methods
Study design
ECHELON-1 is an open-label, international, randomized, phase 3 study of A+AVD vs ABVD in patients with newly diagnosed advanced (stage III or IV) cHL. The study design has been described in detail previously.10 Briefly, patients aged ≥18 years with previously untreated, histologically confirmed stage III/IV cHL were included; patients with nodular lymphocytic predominant HL or with peripheral sensory or motor neuropathy were excluded.
Procedures
Patients were randomized in a 1:1 ratio to receive A+AVD (brentuximab vedotin, 1.2 mg/kg of body weight; doxorubicin, 25 mg/m2 of body-surface area; vinblastine, 6 mg/m2; and dacarbazine, 375 mg/m2) or ABVD (doxorubicin, 25 mg/m2; bleomycin, 10 U/m2; vinblastine, 6 mg/m2; and dacarbazine, 375 mg/m2) IV on days 1 and 15 of each 28-day cycle for up to 6 planned cycles. Patients were stratified according to region (Americas vs Europe vs Asia) and International Prognostic Score (IPS) risk group (low, intermediate, and high risk).
Outcomes
PFS per investigator in the ITT population, an exploratory end point, was evaluated at 3 years. Additional analyses of PFS per investigator, such as those by PET status and age, are post hoc. PFS was defined as time from randomization to the first occurrence of disease progression or death from any cause.11
Statistical analyses
The present analysis reports PFS per investigator in the ITT population, an exploratory end point; PFS analyses for additional subgroups are exploratory and per investigator. The data cutoff date for this analysis was 15 October 2018. Per protocol, 2 formal analyses are prespecified for overall survival. The result of the interim analysis has been published previously10 ; the final overall survival analysis will be performed after 112 deaths have occurred. Survival end points were summarized using the Kaplan-Meier method and evaluated with the use of a log-rank test. Cox regression modeling was used to estimate hazard ratios and confidence intervals (CIs). All P values are nominal and have not been adjusted for multiplicity.
Assessments
PFS per investigator was defined as time from randomization to first documentation of progressive disease (per Cheson et al11 ) or death due to any cause.11,12 Computed tomography and PET scans were obtained at screening and at the end of cycle 2 (PET2). PET2 scans were conducted but were not necessary to determine therapy and were evaluated using the Deauville criteria by central review: PET2− was defined as a Deauville score of 1, 2, or 3, and PET2+ was defined as a Deauville score of 4 or 5. During the follow-up period, computed tomography scans were performed every 3 months for the first year and every 6 months thereafter.
Safety was assessed according to the Medical Dictionary for Regulatory Activities version 19.0,13 and the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.14 Resolution and improvement of PN were monitored during extended follow-up, and all PN events were investigator assessed and reported.
ECHELON-1 was conducted in accordance with regulatory requirements. The protocol was approved by individual institutions’ review boards and ethics committees and adhered to Good Clinical Practice guidelines as defined by the International Conference on Harmonization.
Results
From 19 November 2012 through 13 January 2016, a total of 1334 patients at 218 sites in 21 countries were randomly assigned to receive A+AVD (n = 664) or ABVD (n = 670) (supplemental Figure 1).10 Baseline patient demographics and disease characteristics for the ITT population and by PET2 status were balanced between groups and have been previously described.10 Key data are shown in Table 1.
Key baseline demographics and disease characteristics
| Characteristic . | A+AVD, n = 664 . | ABVD, n = 670 . | Total, N = 1334 . |
|---|---|---|---|
| Male sex | 378 (57) | 398 (59) | 776 (58) |
| Age, y | 35 (18-82) | 37 (18-83) | 36 (18-83) |
| <60 | 580 (87) | 568 (85) | 1148 (86) |
| ≥60 | 84 (13) | 102 (15) | 186 (4) |
| Regions | |||
| Americas | 261 (39) | 262 (39) | 523 (39) |
| Europe | 333 (50) | 336 (50) | 669 (50) |
| Asia | 70 (11) | 72 (11) | 142 (11) |
| IPS | |||
| 0 or 1 | 141 (21) | 141 (21) | 282 (21) |
| 2 or 3 | 354 (53) | 351 (52) | 705 (53) |
| 4 to 7 | 169 (25) | 178 (27) | 347 (26) |
| ECOG performance status | |||
| 0 | 376 (57) | 378 (57) | 754 (57) |
| 1 | 260 (39) | 263 (39) | 523 (39) |
| 2 | 28 (4) | 27 (4) | 55 (4) |
| PET2 status | |||
| Positive | 47 (7) | 58 (9) | 105 (8) |
| Negative | 588 (89) | 577 (86) | 1165 (87) |
| Unknown/unavailable | 29 (4) | 35 (5) | 64 (5) |
| Characteristic . | A+AVD, n = 664 . | ABVD, n = 670 . | Total, N = 1334 . |
|---|---|---|---|
| Male sex | 378 (57) | 398 (59) | 776 (58) |
| Age, y | 35 (18-82) | 37 (18-83) | 36 (18-83) |
| <60 | 580 (87) | 568 (85) | 1148 (86) |
| ≥60 | 84 (13) | 102 (15) | 186 (4) |
| Regions | |||
| Americas | 261 (39) | 262 (39) | 523 (39) |
| Europe | 333 (50) | 336 (50) | 669 (50) |
| Asia | 70 (11) | 72 (11) | 142 (11) |
| IPS | |||
| 0 or 1 | 141 (21) | 141 (21) | 282 (21) |
| 2 or 3 | 354 (53) | 351 (52) | 705 (53) |
| 4 to 7 | 169 (25) | 178 (27) | 347 (26) |
| ECOG performance status | |||
| 0 | 376 (57) | 378 (57) | 754 (57) |
| 1 | 260 (39) | 263 (39) | 523 (39) |
| 2 | 28 (4) | 27 (4) | 55 (4) |
| PET2 status | |||
| Positive | 47 (7) | 58 (9) | 105 (8) |
| Negative | 588 (89) | 577 (86) | 1165 (87) |
| Unknown/unavailable | 29 (4) | 35 (5) | 64 (5) |
Connors et al.10 Values shown are n (%) or median (range).
ECOG, Eastern Cooperative Oncology Group.
In the A+AVD arm, 588 patients (89%) were PET2− and 47 (7%) were PET2+; PET2 status was unknown or unavailable for 29 patients (4%). In the ABVD arm, 577 patients (86%) were PET2− and 58 (9%) were PET2+; PET2 status was unknown or unavailable for 35 patients (5%).
Efficacy
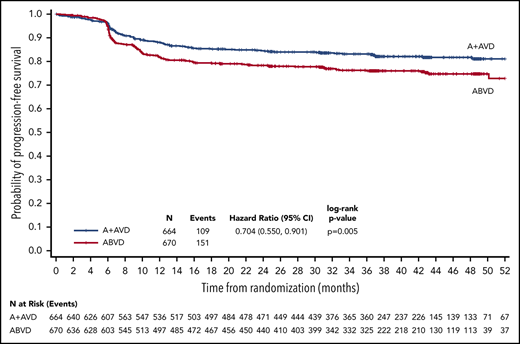
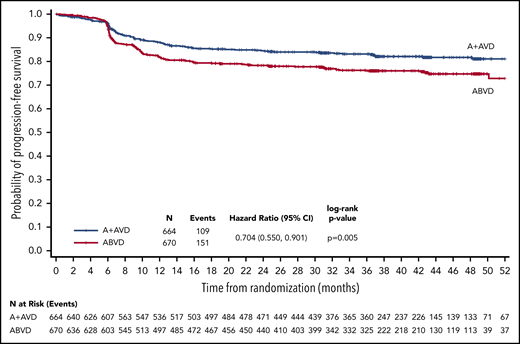
With a median follow-up of 37.1 months (range, 0.0-66.9 months), the 3-year PFS rates per investigator in all patients, irrespective of PET2 status, were 83.1% (95% CI, 79.9-85.9) in the A+AVD arm and 76.0% (95% CI, 72.4-79.2) in the ABVD arm, for a difference of 7.1% favoring the A+AVD arm; the HR was 0.704 (95% CI, 0.550-0.901; P = .005; Figure 1). A PFS improvement at 3 years in patients aged <60 years was also observed (HR = 0.69; P = .008; Table 2). A trend in favor of A+AVD was also observed for the 3-year PFS in the smaller subset of patients aged ≥60 years (HR = 0.79; P = .366; Table 2).
PFS per investigator at 3 years (ITT population). Kaplan-Meier curve of PFS for ITT patients receiving A+AVD or ABVD. Hazard ratio is for 3-year PFS comparison between groups.
PFS per investigator at 3 years (ITT population). Kaplan-Meier curve of PFS for ITT patients receiving A+AVD or ABVD. Hazard ratio is for 3-year PFS comparison between groups.
PFS at 3 years according to PET2 status and age (ITT population)
| . | A+AVD, % (95% CI), n = 664 . | ABVD, % (95% CI), n = 670 . | Difference, % . | HR (95% CI)* . | P† . |
|---|---|---|---|---|---|
| All patients | 83.1 (79.9-85.9) | 76.0 (72.4-79.2) | 7.1 | 0.70 (0.55-0.90) | .005 |
| PET2− | 85.8 (82.6-88.5), n = 577 | 79.5 (75.8-82.7), n = 573 | 6.3 | 0.69 (0.52-0.91) | .009 |
| PET2+ | 67.7 (53.8-78.3), n = 58 | 51.5 (38.2-63.4), n = 63 | 16.2 | 0.59 (0.33-1.07) | .077 |
| Age and PET2 status | |||||
| <60 y | 84.9 (81.6-87.7), n = 580 | 77.8 (73.9-81.1), n = 568 | 7.1 | 0.69 (0.52-0.91) | .008 |
| PET2− | 87.2 (83.9-89.9), n = 512 | 81.0 (77.1-84.4), n = 489 | 6.2 | 0.71 (0.51-0.98) | .034 |
| PET2+ | 69.2 (54.1-80.1), n = 51 | 54.7 (40.0-67.2), n = 54 | 14.5 | 0.60 (0.32-1.15) | .117 |
| ≥60 y | 70.5 (58.6-79.5), n = 84 | 66.5 (55.9-75.2), n = 102 | 4.0 | 0.79 (0.46-1.33) | .366 |
| PET2− | 75.2 (62.1-84.3), n = 65 | 70.9 (59.8-79.5), n = 84 | 4.3 | 0.70 (0.38-1.30) | .258 |
| PET2+ | 57.1 (17.2-83.7), n = 7 | 33.3 (7.8-62.3), n = 9 | 23.8 | 0.65 (0.16-2.63) | .545 |
| . | A+AVD, % (95% CI), n = 664 . | ABVD, % (95% CI), n = 670 . | Difference, % . | HR (95% CI)* . | P† . |
|---|---|---|---|---|---|
| All patients | 83.1 (79.9-85.9) | 76.0 (72.4-79.2) | 7.1 | 0.70 (0.55-0.90) | .005 |
| PET2− | 85.8 (82.6-88.5), n = 577 | 79.5 (75.8-82.7), n = 573 | 6.3 | 0.69 (0.52-0.91) | .009 |
| PET2+ | 67.7 (53.8-78.3), n = 58 | 51.5 (38.2-63.4), n = 63 | 16.2 | 0.59 (0.33-1.07) | .077 |
| Age and PET2 status | |||||
| <60 y | 84.9 (81.6-87.7), n = 580 | 77.8 (73.9-81.1), n = 568 | 7.1 | 0.69 (0.52-0.91) | .008 |
| PET2− | 87.2 (83.9-89.9), n = 512 | 81.0 (77.1-84.4), n = 489 | 6.2 | 0.71 (0.51-0.98) | .034 |
| PET2+ | 69.2 (54.1-80.1), n = 51 | 54.7 (40.0-67.2), n = 54 | 14.5 | 0.60 (0.32-1.15) | .117 |
| ≥60 y | 70.5 (58.6-79.5), n = 84 | 66.5 (55.9-75.2), n = 102 | 4.0 | 0.79 (0.46-1.33) | .366 |
| PET2− | 75.2 (62.1-84.3), n = 65 | 70.9 (59.8-79.5), n = 84 | 4.3 | 0.70 (0.38-1.30) | .258 |
| PET2+ | 57.1 (17.2-83.7), n = 7 | 33.3 (7.8-62.3), n = 9 | 23.8 | 0.65 (0.16-2.63) | .545 |
HRs (A+AVD/ABVD) and 95% CIs are based a Cox proportional hazard regression model, which is stratified for the ITT population and unstratified for subgroup analyses.
P values are calculated using a log-rank test, which is stratified for the ITT population and unstratified for subgroup analyses.
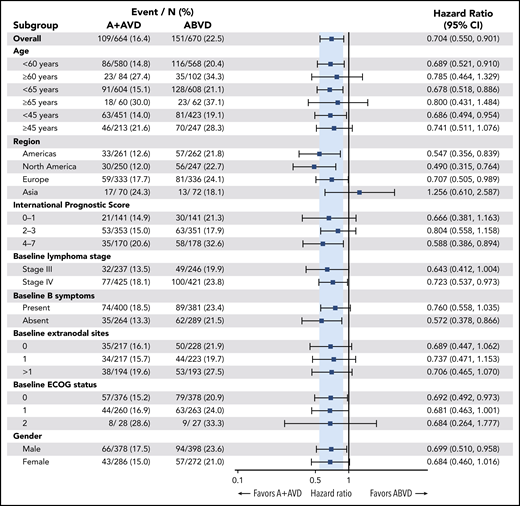
Consistent improvement in PFS per investigator was observed in patients treated with A+AVD vs those treated with ABVD across nearly all prespecified subgroups in the ITT population, including key subgroups such as stage III disease, stage IV disease, age, and IPS (Figure 2). A trend toward efficacy benefit with A+AVD was also observed in patients aged <60 years independent of IPS risk group, including IPS 0-1 (HR = 0.59; P = .071), IPS 2-3 (HR = 0.79; P = .286), and IPS 4-7 (HR = 0.64; P = .083).
Forest plot of 3-year PFS per investigator. Forest plot of 3-year PFS for patient subgroups indicating favored treatment of each subgroup. ECOG, Eastern Cooperative Oncology Group.
Forest plot of 3-year PFS per investigator. Forest plot of 3-year PFS for patient subgroups indicating favored treatment of each subgroup. ECOG, Eastern Cooperative Oncology Group.
Safety
At the 3-year follow-up analysis, 78% of patients (345 of 442) with PN in the A+AVD arm had either complete resolution (62% [272 of 442]) or improvement (17% [73 of 242]) of PN, compared with 83% (236 of 286) with either complete resolution (73% [209 of 286]) or improvement (9% [27 of 286]) in the ABVD arm. In the A+AVD arm, 170 patients (25%) had ongoing PN after continued follow-up, of whom 152 (89%) had ongoing grade 1/2 events; 99 and 53 patients had ongoing grade 1 and 2 events, respectively. In the ABVD arm, 77 patients (11%) had ongoing PN after continued follow-up, of whom 73 (95%) had ongoing grade 1/2 events; 49 and 24 patients had ongoing grade 1 and 2 events, respectively.
The median time to complete resolution of PN following the end of A+AVD or ABVD treatment was 28 weeks (range, 0-167 weeks) and 14 weeks (range, 0-188 weeks), respectively. In patients whose PN did not completely resolve, the median time to improvement of PN following end of treatment was 40 weeks (range, 8-129 weeks) in the A+AVD arm and 32 weeks (range, 2-70 weeks) in the ABVD arm.
By 3 years, 34 patients developed secondary malignancies, including 14 in the A+AVD arm (6 solid, 8 hematologic) and 20 in the ABVD arm (9 solid, 11 hematologic).
Discussion
This updated analysis of ECHELON-1 demonstrated that with a median of 3 years of follow-up, frontline treatment of patients with stage III or IV cHL with 6 cycles of A+AVD provided a robust, sustained, efficacy benefit vs ABVD that was independent of PET2 status. The 3-year PFS with A+AVD was 83.1% (95% CI, 79.9% to 85.9%) compared with 76.0% (95% CI, 72.4% to 79.2%) in the ABVD arm, a difference of 7.1% (HR = 0.70; P = .005). A consistent efficacy benefit was also supported by the analysis of prespecified subgroups, including both stage III and IV patients, patients aged <60 years, and all patients independent of IPS risk group. PN continued to completely resolve or improve in patients in both the A+AVD and ABVD arms, and residual PN was low grade.
PET-adapted treatment strategies for the management of patients receiving frontline treatment of advanced HL were designed with the intent to maintain efficacy and minimize short- and long-term toxicities. This approach requires real-time disease assessment at cycle 2, which can be challenging in some practice settings. Although caution should be used when comparing results across studies, including differences in study population (ECHELON-1 excluded patients with stage II disease and included patients aged ≥60 years) among other factors, placing the results of the ECHELON-1 study in the context of PET-adapted treatment options is valuable. The RATHL trial, which enrolled 42% of patients with stage II HL, randomized PET2− patients (84%) after 2 cycles of ABVD to additional ABVD or de-escalation to AVD, and escalated PET2+ patients to BEACOPP. Although the use of AVD vs ABVD did not exclude the difference of 5% required to accept noninferiority at 3 years in PET2− patients (PFS rates: ABVD, 85.7%; AVD, 84.4%) (95% CI, −3.2 to 5.3), the authors conclude that the omission of bleomycin after cycle 2 carries a minimal risk of treatment failure and the difference is not clinically significant. Patients whose treatment was escalated to 4 cycles of BEACOPP (BEACOPP-14 or BEACOPPescalated) had a 3-year PFS of 67.5%, similar to the rates seen in other studies that used PET2-adapted escalation to BEACOPP.5,6 Additionally, among patients aged ≤60 years of age with stage III/IV disease, PET2− patients who received ABVD/AVD had an overall 3-year PFS rate of 82.1% whereas PET2+ patients whose treatment was escalated to BEACOPP had a 3-year PFS rate of 63.9%.5
Other strategies incorporating the use of targeted agents have been reported, including the GITIL/FIL HD 0607 and CheckMate 205 trials investigating the use of rituximab or nivolumab, respectively.6,15 The GITIL/FIL HD 0607 trial, in which 36% of patients had stage II disease, studied 2 cycles of ABVD followed by escalation to BEACOPP or BEACOPP with rituximab in PET2+ patients while PET2− patients (81%) continued ABVD.6 At the end of the treatment, PET2− patients with a large nodal mass at diagnosis (≥5 cm) were randomized to receive consolidative radiotherapy or no further treatment. At 3 years, PET2− and PET2+ patients had PFS rates of 87% and 60%, respectively. Neither the addition of rituximab to BEACOPP nor the addition of consolidative radiotherapy significantly improved outcomes.6 Additionally, the phase 2 CheckMate-205 cohort D study investigated 4 doses of nivolumab (N) monotherapy followed by 12 doses of N+AVD in 51 patients with previously untreated, advanced-stage cHL (20% stage IIB).15 At the end of treatment, the objective response rate was 84%; however, the median follow-up period was only 9.4 months, with a 9-month modified PFS of 92%.15 With the small sample size and short follow-up period, additional data are necessary to establish the safety and efficacy of N+AVD. A phase 3 study (SWOG S1826; NCT03907488) comparing the combination of A+AVD vs N+AVD in patients with previously untreated, stage III or IV cHL was recently initiated.
Although trials that assessed the safety and efficacy of PET-adapted strategies have resulted in improvements for patients whose disease failed to adequately respond to frontline therapy, emerging data from the SWOG S0816 trial suggest that long-term outcomes in both PET2+ and PET2− patients may not be as robust as initially anticipated. Patients with stage III or IV disease received 2 cycles of ABVD and either continued with an additional 4 cycles if PET2− or escalated to up to 6 of BEACOPP if PET2+. At 2 years, PFS was 79% for all patients, and 64% for PET2+ patients.9 Importantly, the 5-year follow-up of SWOG S0816, which studied continued ABVD for PET2− or escalation to BEACOPP for PET2+ in patients aged ≤60 years with stage III and IV disease, showed an unexpectedly high 24% rate of relapse in PET2− patients treated with ABVD.7 In addition, high rates of secondary malignancies (14%) were seen among interim PET2+ patients whose treatment was escalated to 6 cycles of BEACOPP.
The goals of PET-directed therapy include mitigation of adverse events, including bleomycin-related pulmonary toxicity. The 2-year analysis of ECHELON-1 showed that fewer patients receiving A+AVD experienced pulmonary toxicities (2%) compared with patients in the ABVD arm (7%), with no deaths due to or associated with pulmonary toxicity; in the ABVD arm, 85% of deaths (11 of 13) during treatment were due to or associated with pulmonary toxicity. In RATHL, there was a trend toward decreased adverse events in patients whose treatment was de-escalated to AVD compared with patients who remained on ABVD, including a significant decrease in the rate of pulmonary toxicity after cycle 2 (1% vs 3%, respectively).5 Pulmonary toxicity has been observed in patients even after the first and second doses of bleomycin, highlighting the importance of monitoring for bleomycin-related complications even when limiting exposure.16
Intensified chemotherapy regimens such as BEACOPPescalated have also been associated with an increased risk of secondary malignancies.17 In the primary analysis of SWOG S0816, 6% of PET2+ patients with escalation to 6 cycles of BEACOPP reported secondary malignancies, which increased to 14% with 5.9 years of follow-up.7,9 In RATHL, at 3 years of follow-up, 2.4% of patients developed secondary malignancies (ABVD arm, 2.8%; AVD arm, 2.4%; BEACOPP arm, 1.7%).5 At a median follow-up of 3.6 years, no secondary malignancies were seen in the GITIL/FIL HD 0607 trial.6 With 3 years of follow-up, the rates of secondary malignancies in ECHELON-1 were 2.3% in the A+AVD arm and 3% in the ABVD arm. These preliminary results suggest that the risk of secondary malignancies with A+AVD does not exceed that with ABVD, although it is too early for a definitive conclusion. Follow-up of patients in ECHELON-1 is ongoing.
At the time of the primary analysis, the adverse event profile of A+AVD was consistent with the individual components of the regimen. With A+AVD vs ABVD, a higher rate of neutropenia, including febrile neutropenia and PN, and a lower rate of pulmonary toxicity was observed. Although treatment with A+AVD vs ABVD was associated with a higher rate of febrile neutropenia (19% vs 11%), the rate was reduced and comparable (11%) to ABVD among patients who received A+AVD with G-CSF primary prophylaxis beginning with cycle 1, highlighting the importance of G-CSF primary prophylaxis for all patients treated with A+AVD. At the 3-year follow-up, PN continued to resolve and improve in both arms, with 78% and 83% of patients in the A+AVD and ABVD arms experiencing resolution or improvement, respectively. This represents an 11% improvement for the A+AVD group and 8% for the ABVD group compared with 2-year follow-up results.10 Although ongoing PN was reported by the investigators in 25% of patients in the A+AVD arm and in 11% of patients in the ABVD arm, the majority of ongoing PN events were grade 1 or 2. The incidence, severity, and rate of recovery of PN in adolescents and young adults and patients aged ≥60 years receiving A+AVD were similar to the ITT population at 2 years, with the exception of a higher rate of grade 3 PN in patients ≥60 years of age.18,19 No additional long-term toxicities have been observed.
Taken together, data presented in this 3-year analysis of ECHELON-1 suggest that A+AVD compares favorably to ABVD and PET-adapted strategies without requiring a change of therapy based on interim PET assessment while eliminating exposure to bleomycin. A+AVD provided a sustained PFS benefit with a predictable and manageable safety profile, with continued complete resolution and improvement of PN. These data further support the advantages of A+AVD vs ABVD as frontline treatment of patients with advanced-stage III or IV cHL.
Requests for original data should be sent to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Medical writing assistance was provided by Michael R. Convente (ScientificPathways, Inc) with funding from Seattle Genetics, Inc.
This work was supported by Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, and Seattle Genetics, Inc. The trial was designed by representatives of Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, and Seattle Genetics with guidance from a committee of 6 academic authors.
Data were collected and trial procedures were overseen by the trial investigators. Data were verified and analyzed by the sponsors and sponsor statisticians and interpreted by academic authors and representatives of Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, and Seattle Genetics.
Authorship
Contribution: D.J.S., M.D.-D., S.A., Á.I., M.P., E.L.-M., T.F., P.S., K.J.S., N.L.B., J.W., R.R., P.L.Z., M.H., J.M.C., J.R., J.M., W.S.K., R.A., S.M.A., A.Y., A.F.-T., and A.G. participated in data collection; K.F. and R.L. performed data analysis; D.J.S., H.M., R.L., K.F., and A.F.-T. performed data interpretation and drafted the manuscript; and all authors reviewed the manuscript and provided final approval.
Conflict-of-interest disclosure: D.J.S. reports research funding from, and serves as a consultant for, Seattle Genetics, and received honoraria for journal article commentaries for Elsevier PracticeUpdate. E.L.-M. reports advisory board personal fees from Takeda outside of the submitted work. T.F. reports the following outside of the submitted work: consultancy on the advisory boards for Seattle Genetics, Bristol-Myers Squibb, and Celgene; and honoraria from, and speakers’ bureaus for, Takeda, Celgene, Seattle Genetics, AbbVie, Pharmacyclics, Janssen, KITE, and Bristol-Myers Squibb. P.S. reports clinical meeting support from Roche and Takeda, and personal fees from Takeda outside of the submitted work. K.J.S. reports acting in a consulting or advisory role for Seattle Genetics, Bristol-Myers Squibb, Merck, Servier, Novartis, AbbVie, and Verastem; honoraria from Seattle Genetics, Bristol-Myers Squibb, Merck, Novartis, Takeda, and Astra Zeneca; and research funding from Roche. N.L.B. reports the following, all outside of the submitted work: research funding from Gilead, Immune Design, Incyte, Janssen, MedImmune, Merck, Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, Novartis, and Pharmacyclics; research funding from, and advisory board service for, Kite Pharma and Seattle Genetics; and advisory board service for BTG, Acerta, and Pfizer. J.W. reports the following, all outside of the submitted work: research funding and investigator fees from Seattle Genetics and Takeda; research grants, a consultancy agreement, lecture honoraria, and travel grants from Roche; a consultancy agreement and lecture honoraria from Celgene; research grants, consultancy agreements, and lecture honoraria from Takeda and Janssen-Cilag; consultancy agreements and lecture honoraria from Servier and Amgen; consultancy agreements from Bristol-Myers Squibb, Incyte, and AbbVie; research grants from GlaxoSmithKline/Novartis; and consultancy agreements on the advisory boards from Gilead and Novartis. R.R. reports consultancy for Seattle Genetics outside of the submitted work. P.L.Z. reports consultancy for Verastem, Merck Sharp & Dohme, Eusapharma, and Sanofi; and has served on speakers’ bureaus and advisory boards for Verastem, Celltrion, Gilead, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, Merck Sharp & Dohme, Celgene, Portola, Roche, Eusapharma, Kyowa Kirin, and Sanofi. M.H. reports research funding and study fees from Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited; and advisory board service and consultancy for, and research support from, Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, outside of the submitted work. J.M.C. reports research support from Seattle Genetics, and honoraria from Seattle Genetics and Takeda. J.R. reports consultancy for and personal fees from ADC Therapeutics, Takeda, Bristol-Myers Squibb, and Novartis outside of the submitted work, and his spouse owns stock in AstraZeneca and GlaxoSmithKline. J.M. reports grants from, consultancy for, research funding from, and speakers’ bureau service for Pharmacylics and Gilead/Kite; consultancy and speaker’s bureau service for Bayer; consultancy for Pfizer and Alexion; grants from, consultancy for, and research funding from Janssen, Juno, Celgene, and Bristol-Myers Squibb; personal fees from, consultancy for, honoraria from, and speaker’s bureau service for Kyowa; grants and personal fees from, consultancy for, and honoraria and research funding from Seattle Genetics; grants and research funding from Portola, Incyte, and Genentech; and speakers’ bureau service for Fosunkite and AstraZeneca. W.S.K. reports research funding from Roche, Pfizer, Johnson and Johnson, Takeda, Mundypharma, Celltrion, Kyowa-Kirin, and Donga. R.A. reports the following, all outside of the submitted work: advisory role consulting fees from AstraZeneca, Autolus, Bayer Healthcare Pharmaceuticals, Cell Medica, Gilead, Kite Pharma, Kyowa, and Takeda; advisory role consulting fees and institutional research support from Genentech/Roche, Pharmacyclics, and Seattle Genetics; and institutional research support from Agensys, Celgene, Forty Seven, Inc, Janssen Pharmaceutical, Kura, Merck, Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceuticals Company Limited, and Regeneron. S.M.A. reports research funding for the trial from Takeda; and funding for the trials from Bristol-Myers Squibb, Affimed, Regeneron, and AI Therapeutics outside of the submitted work. A.Y. reports the following, all outside of the submitted work: research support grants from Janssen, Curis, Merck, Bristol-Myers Squibb, Syndax, and Roche; honoraria from Janssen, Abbvie, Merck, Curis, Epizyme, Roche, and Takeda; and consulting fees from Biopath, Xynomics, Epizyme, Roche, Celgene, and HCM. H.M. and R.L. report employment with Takeda. K.F. and A.F.-T. are employees at Seattle Genetics and own equity in the company. A.G. reports receipt of honoraria from Takeda. The remaining authors declare no competing financial interests.
Correspondence: David J. Straus, Lymphoma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065, e-mail: strausd@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal