Abstract
For patients with venous thromboembolism (VTE), prediction of bleeding is relevant throughout the course of treatment, although the means and goal of this prediction differ between the subsequent stages of treatment: treatment initiation, hospital discharge, 3-month follow-up, and long-term follow-up. Even in the absence of fully established risk prediction schemes and outcome studies using a prediction scheme for treatment decisions, the present evidence supports screening for and targeting of modifiable risk factors for major bleeding, as well as the application of decision rules to identify patients at low risk of bleeding complications, in whom long-term anticoagulant treatment is likely safe. Moving forward, prediction tools need to be incorporated in well-designed randomized controlled trials aiming to establish optimal treatment duration in patients at high risk of recurrent VTE. Moreover, the benefit of their longitudinal assessment rather than application as stand-alone baseline assessments should be studied, because changes in bleeding risk over time likely constitute the best predictor of major bleeding. We provide the state-of-the-art of assessing and managing bleeding risk in patients with acute VTE and highlight a practical approach for daily practice illustrated by 2 case scenarios.
Introduction
The most feared complication of anticoagulant treatment of venous thromboembolism (VTE) is major bleeding, which occurs up to a rate of 7.22 per 100 patient years, depending on the prescribed anticoagulant drug class, with a case fatality rate of ∼9%.1 This risk of bleeding is particularly high in the first months of anticoagulant treatment and when patients are receiving thrombolytic treatment. Notably, the consequences of (temporarily) anticoagulant discontinuation in this period are more serious than during long-term treatment because of the higher risk of recurrent VTE.2 Clinical guidelines on the treatment of VTE therefore propose incorporating assessment of bleeding in treatment decisions, although simple, validated tools to do so are not provided.3,4 It is suggested to either use implicit judgment after evaluating individual risk factors or, alternatively, apply a bleeding risk scheme prior to starting reperfusion treatment or initiating anticoagulant treatment.3,4 This recommendation may however be regarded as an oversimplified view on the often complex management of risk of bleeding in daily practice. Particularly, bleeding risk assessment can be used to (1) reduce the overall risk of bleeding by targeting identified risk factors, but also to (2) determine the optimal anticoagulant drug class, (3) determine the optimal drug dose, and (4) determine the optimal treatment duration.5,6 Importantly, it is unlikely that the same stratification tools can be used for all these different purposes. Moreover, risk profiles may change over time, and especially sudden changes of risk factors can cause a drastic transition between risk classes and contribute largely to the risk of major bleeding events. Hence, the optimal management of the bleeding risk in VTE patients should consist of a careful interplay between periodically assessed individual risk factors and application of validated multivariable stratification tools. In this review, we provide the state-of-the-art assessment and management of bleeding risk in patients treated for acute VTE. We highlight a practical approach for daily practice illustrated by 2 case scenarios. Because patients with cancer-associated VTE constitute a unique population requiring a specific approach, risk prediction in these patients will not be covered by this review.
Case presentations
Scenario 1
A 45-year-old man is diagnosed with deep vein thrombosis (DVT) 3 days after transsphenoidal adenectomy of a macroprolactinoma. He has a history of hypercholesterolemia and myocardial infarction 4 years ago, for which he underwent a percutaneous coronary intervention and received 2 drug-eluting stents. His preoperative medication included a statin, a β-blocker, low-dose aspirin, and an angiotensin receptor blocker. After he developed painful swelling of his right leg, compression ultrasound revealed proximal DVT extending to the external femoral vein. You are consulted to determine the treatment of the DVT.
Questions to consider
Is it safe to start anticoagulant therapy shortly after surgery?
What anticoagulant strategy has the lowest risk of bleeding?
Which modifiable risk factors for bleeding can be identified, and how should these be managed?
Scenario 2
A 41-year-old woman is diagnosed with unprovoked low-risk pulmonary embolism (PE). Her history is notable for type 1 diabetes mellitus, which is complicated by retinopathy, nephropathy (current stable estimated glomerular filtration rate 55 mL/min), and hypertension. Her medication includes insulin, a statin, a calcium channel blocker, an angiotensin-converting-enzyme inhibitor, and a thiazide diuretic. You are consulted to determine the treatment of the PE.
Questions to consider
Which modifiable risk factors for bleeding can be identified, and how should these be managed?
What anticoagulant strategy has the lowest risk of bleeding?
Which considerations are relevant to determine the duration of anticoagulant therapy?
Prediction of bleeding in patients with VTE
At initiation of treatment
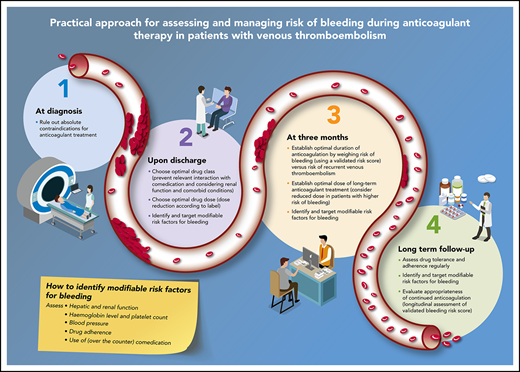
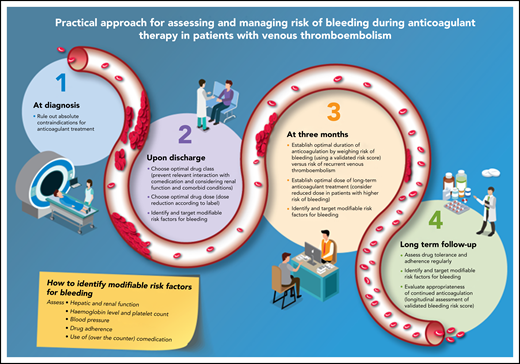
The first assessment of bleeding risk should be performed at the time of the VTE diagnosis to minimize the risk of bleeding during the obligatory initiation of anticoagulation (Figure 1): therapeutically dosed anticoagulation is indicated for a period of at least 3 months in all patients with newly diagnosed acute VTE.3,4 In general, anticoagulant treatment for preventing recurrent VTE and related mortality outweighs bleeding risk in most situations. Patients may however have an absolute contraindication to therapeutic doses of anticoagulant treatment: anticoagulant treatment in these patients should be avoided. Absolute contraindications include active intracranial bleeding or other life-threatening bleeding, recent exposure to major surgical interventions, and thrombocytopenia <30 × 109/L. Although risk stratification instruments have never been developed for this initial setting, common sense dictates that anticoagulant therapy should be withheld for the duration of the contraindication, for example, until hemostasis is achieved as decided in conjunction with the multidisciplinary team of responsible physicians.7-11 Alternative treatment strategies in patients with an acute DVT or PE but an absolute contraindication to anticoagulation could involve the use of a retrievable vena cava filter, (repeated) platelet transfusion to deal with “critical” thrombocytopenia, and/or temporarily prophylactic anticoagulation.3,4 Restarting anticoagulation when the contraindication has resolved requires careful reassessment of the risks and benefits.
Practical approach to assess and manage risk of bleeding in patients with VTE. (1) At diagnosis; (2) upon discharge; (3) at 3 months; and (4) long term follow-up.
Practical approach to assess and manage risk of bleeding in patients with VTE. (1) At diagnosis; (2) upon discharge; (3) at 3 months; and (4) long term follow-up.
Predicting the risk of bleeding is also relevant for the setting of high-risk VTE in which reperfusion therapy is indicated,3,4 that is, patients with PE who are hemodynamically unstable, or patients with massive iliofemoral thrombosis (phlegmasia cerulea dolens) with compromised arterial circulation. In the absence of large trials with alternative treatment strategies, full-dose systemic thrombolytic therapy remains the standard of care in such patients, which is associated with a 9.9% incidence of major bleeding and 1.7% incidence of intracerebral or fatal bleeding.12 Guidelines provide lists of absolute and relative contraindications to fibrinolysis (Table 1), although terminology is vague (“bleeding diathesis”) and application remains difficult.3,4 Dedicated schemes to standardize risk stratification in this setting have been developed. The PE-CH (peripheral vascular disease, elderly, prior cerebrovascular accident [CVA], and prior Heart attack) score was developed by identifying 4 independent prognostic factors: preexisting peripheral vascular disease, age >65 years, prior CVA with residual deficit, and prior myocardial infarction (Table 1). In the derivation cohort, scores of 0, 1, 2, and 5 points were associated with intracranial hemorrhage risks of 1.2%, 1.9%, 2.4%, and 17.8%, respectively; the rates of intracranial hemorrhage were similar in the validation cohort. The C-statistic for the risk score was 0.65 (0.61 to 0.70) in the derivation cohort and 0.66 (0.60 to 0.72) in the validation. The main drawbacks are the moderate discriminatory capability and lack of prospective evaluation in outcome studies.13 Alternative treatment strategies other than systemic thrombolysis in patients with perceived unacceptable risk of bleeding involve surgical thrombectomy or percutaneous catheter-directed treatment, with or without support by extracorporeal membrane oxygenation in high-risk PE patients.3,4 Notably, percutaneous catheter-directed treatment is also associated with a risk of major bleeding, especially at the puncture site.14,15
Risk stratification for thrombolysis
| Contraindications as provided in European Society of Cardiology guidelines4 . | Contraindications as provided in ACCP guidelines3 . | Peripheral vascular disease, elderly, prior CVA, and prior heart attack score13 . |
|---|---|---|
Absolute contraindications
| Major contraindications
|
|
Relative contraindications
| Relative contraindications
|
| Contraindications as provided in European Society of Cardiology guidelines4 . | Contraindications as provided in ACCP guidelines3 . | Peripheral vascular disease, elderly, prior CVA, and prior heart attack score13 . |
|---|---|---|
Absolute contraindications
| Major contraindications
|
|
Relative contraindications
| Relative contraindications
|
ACCP, American College of Chest Physicians.
Upon discharge
The moment of hospital discharge, which may be the same day as the moment of diagnosis, necessitates further risk stratification. First, the optimal anticoagulant drug class and dose should be identified, not only aimed at patient preference or health economic considerations, but especially also to expose the patient to an effective drug (dose) associated with the lowest risk of bleeding. Second, modifiable risk factors for bleeding should be identified and targeted, to reduce the risk of incident major bleeding complications. Importantly, this stage of risk management involves the complete first treatment period up to the 3-month follow-up visit and overlaps with the stage “at initiation of treatment,” especially in low-risk patients treated at home.
In recent years, several drug classes can be prescribed to patients with VTE for the initial weeks of treatment: low-molecular-weight heparin (LMWH), vitamin K antagonists (VKA), and direct oral anticoagulants (DOACs). Large phase 3 study programs have demonstrated that DOAC therapy is associated with a lower risk of major, intracranial, and fatal bleeding than VKA therapy, making DOACs the treatment of choice.3,4,6,16 In several settings though, VKA is still preferred over DOACs to prevent major bleeding, for example, in case of poor compliance to therapy or relevant interaction with comedication. The metabolism and excretion of DOACs, dependent on the individual drug, may be modulated by the enzymes CYP3A4, CYP2J2, and P-glycoprotein. When combined with inhibitors of these enzymes, such as antimycotics and HIV protease inhibitors, the pharmacokinetic activity of DOACs is altered, exposing patients at increased risk of bleeding.17 Therefore, DOACs need to be described according to their label, with dose reduction where relevant and avoiding relevant medication interactions.
The first weeks of anticoagulant treatment involve the highest incidences of bleeding in anticoagulation-naive patients, with a twofold higher incidence in the first 3 months than during long-term treatment.1,5,18,19 The initial treatment period is thus one of the most relevant ones to apply bleeding prevention strategies, which mainly consists of identifying and targeting modifiable risk factors, for example, by treating hypertension, minimizing the duration and intensity of concomitant antiplatelet and nonsteroidal anti-inflammatory drug therapy, eliminating causes for blood loss, moderating alcohol intake, and overcoming intercurrent and reversible renal and hepatic disease (Table 2; Figure 1).3,4 Randomized controlled trials to prove that this will indeed reduce the incidence of bleeding are not, and will never become, available for obvious reasons. However, from studies in atrial fibrillation (AF), it has been shown that successful targeting of modifiable risk factors can be achieved by relatively simple interventions and lead to permanent reclassification of the bleeding risk, with the change in risk class being the best predictor of outcome.20,21 Moreover, modifiable risk factors per se have been shown to be poor predictors for major bleeding relative to established risk stratification schemes, probably because these can been reversed.21,22 Where it has been shown that a decline in estimated glomerular filtration rate, even to a small degree, is associated with an increased risk of bleeding,23,24 improvement of renal function typically is related to a decreased incidence of anticoagulant-associated major bleeding.25 Furthermore, it has been established that advancing stages of hypertension during antithrombotic medication are associated with higher incidences of intracranial bleeding and bleeding in general.26,27 The well-known steady increase of the risk of bleeding with increasing blood pressures is suggestive of a causal relation, making it likely that lowering blood pressures will lead to mitigation of the risk of bleeding.
Proposed risk stratification schemes to predict bleeding in patients with VTE
| . | ACCP risk table3 . | VTE-BLEED19 . | Kuijer32 . | RIETE33 . | Einstein model35 . | Hokusai model34 . | Nieuwenhuis36 . | Score according to Seiler37 . |
|---|---|---|---|---|---|---|---|---|
| Risk factors | ||||||||
| Age | X | |||||||
| Age ≥60 y | 1.5 points | 1.6 points | ||||||
| Age >65 y | X | |||||||
| Age >75 y | X | 1 point | ||||||
| WHO grade 1 | 1 point | |||||||
| WHO grade 2 to 3 | 2 points | |||||||
| Low physical activity* | 2 points | |||||||
| Previous bleeding | X | 1.5 points | 2 points | 1 point | ||||
| Recent major bleeding | 2 points | |||||||
| Active cancer* | X | 2 points | 2.2 points | 1 point | ||||
| Metastatic cancer | X | |||||||
| Renal failure/insufficiency* | X | 1.5 points | 1.5 points | |||||
| Liver failure* | X | |||||||
| Thrombocytopenia* | X | 1 point | ||||||
| Previous stroke | X | |||||||
| Diabetes* | X | |||||||
| Anemia* | X | 1.5 points | 1.5 points | 1 point | 1 point | |||
| Hemoglobin level* | X | |||||||
| Antiplatelet therapy* | X | 1 point | 1 point | |||||
| Poor anticoagulant control* | X | 1 point | ||||||
| Comorbidity and reduced functional capacity | X | |||||||
| Recent trauma or surgery | X | 1 point | ||||||
| Frequent falls* | X | |||||||
| Alcohol abuse* | X | |||||||
| Nonsteroidal anti-inflammatory drug* | X | |||||||
| Male patient with uncontrolled hypertension* | 1 point | X | ||||||
| History of hypertension* | 1 point | |||||||
| Blood pressure >160 mmHg* | 1 point | |||||||
| Male patient with anemia* | X | |||||||
| History of cardiovascular disease | X | |||||||
| Body surface area <2 m2 | 2 points | |||||||
| Female sex | 1.3 points | 1 point | ||||||
| Black or Asian race | X | |||||||
| Clinically overt PE | 1 point | |||||||
| Risk stratification | ||||||||
| Low risk | 0 risk factors | <2 points | 0 points | 0 points | Model provided by the authors | No threshold provided | 0 to 2 points | 0 to 1 points |
| Intermediate risk | 1 risk factor | 1.3 to 2.9 points | 1 to 4 points | 2 to 4 points | 2 to 3 points | |||
| High risk | ≥2 risk factors | ≥2 points | >2.9 points | >4 points | ≥5 points | ≥4 points | ||
| Bleeding event | ||||||||
| Definition of major bleeding | N/A | ISTH major77 | ≥2 g/dL drop in hemoglobin, requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal location, or warranting permanent treatment discontinuation | Investigator-reported overt bleeding requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal or spinal location, or leading to death | ISTH major77 | ISTH major77 | Bleeding that leads to death, to interruption of treatment, to blood transfusion, or to a decrease in hemoglobin level of >2.42 g/dL (1.5 mmol/L) | ISTH major77 |
| Bleeding events formally adjudicated | N/A | Yes | Yes | No | Yes | Yes | No | Yes |
| . | ACCP risk table3 . | VTE-BLEED19 . | Kuijer32 . | RIETE33 . | Einstein model35 . | Hokusai model34 . | Nieuwenhuis36 . | Score according to Seiler37 . |
|---|---|---|---|---|---|---|---|---|
| Risk factors | ||||||||
| Age | X | |||||||
| Age ≥60 y | 1.5 points | 1.6 points | ||||||
| Age >65 y | X | |||||||
| Age >75 y | X | 1 point | ||||||
| WHO grade 1 | 1 point | |||||||
| WHO grade 2 to 3 | 2 points | |||||||
| Low physical activity* | 2 points | |||||||
| Previous bleeding | X | 1.5 points | 2 points | 1 point | ||||
| Recent major bleeding | 2 points | |||||||
| Active cancer* | X | 2 points | 2.2 points | 1 point | ||||
| Metastatic cancer | X | |||||||
| Renal failure/insufficiency* | X | 1.5 points | 1.5 points | |||||
| Liver failure* | X | |||||||
| Thrombocytopenia* | X | 1 point | ||||||
| Previous stroke | X | |||||||
| Diabetes* | X | |||||||
| Anemia* | X | 1.5 points | 1.5 points | 1 point | 1 point | |||
| Hemoglobin level* | X | |||||||
| Antiplatelet therapy* | X | 1 point | 1 point | |||||
| Poor anticoagulant control* | X | 1 point | ||||||
| Comorbidity and reduced functional capacity | X | |||||||
| Recent trauma or surgery | X | 1 point | ||||||
| Frequent falls* | X | |||||||
| Alcohol abuse* | X | |||||||
| Nonsteroidal anti-inflammatory drug* | X | |||||||
| Male patient with uncontrolled hypertension* | 1 point | X | ||||||
| History of hypertension* | 1 point | |||||||
| Blood pressure >160 mmHg* | 1 point | |||||||
| Male patient with anemia* | X | |||||||
| History of cardiovascular disease | X | |||||||
| Body surface area <2 m2 | 2 points | |||||||
| Female sex | 1.3 points | 1 point | ||||||
| Black or Asian race | X | |||||||
| Clinically overt PE | 1 point | |||||||
| Risk stratification | ||||||||
| Low risk | 0 risk factors | <2 points | 0 points | 0 points | Model provided by the authors | No threshold provided | 0 to 2 points | 0 to 1 points |
| Intermediate risk | 1 risk factor | 1.3 to 2.9 points | 1 to 4 points | 2 to 4 points | 2 to 3 points | |||
| High risk | ≥2 risk factors | ≥2 points | >2.9 points | >4 points | ≥5 points | ≥4 points | ||
| Bleeding event | ||||||||
| Definition of major bleeding | N/A | ISTH major77 | ≥2 g/dL drop in hemoglobin, requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal location, or warranting permanent treatment discontinuation | Investigator-reported overt bleeding requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal or spinal location, or leading to death | ISTH major77 | ISTH major77 | Bleeding that leads to death, to interruption of treatment, to blood transfusion, or to a decrease in hemoglobin level of >2.42 g/dL (1.5 mmol/L) | ISTH major77 |
| Bleeding events formally adjudicated | N/A | Yes | Yes | No | Yes | Yes | No | Yes |
ISTH, International Society on Thrombosis and Haemostasis; NA, not applicable.
Potentially modifiable risk factors.
Considering the obligatory 3-month treatment with full-dose anticoagulation for all VTE patients, speculation on duration and dosing (other than dose reductions recommended by the label) of anticoagulation is still premature at this point in time. Hence, application of risk schemes to identify patients at higher risk of bleeding is of little additional benefit, because meaningful interventions other than treating modifiable risk factors are not available.
After 3 months
At the routine 3-month follow-up visit, the duration of anticoagulation should be established, a decision mainly based on the risk of recurrent VTE in the case of anticoagulant discontinuation (Figure 1).3,4,28-30 The risk of bleeding is only relevant for those patients at high risk of recurrent VTE, that is, those with unprovoked VTE or VTE associated with minor persistent or transient risk factors, in whom indefinite anticoagulation is to be considered. The risk of anticoagulant-associated bleeding should be weighed against the risk of recurrent VTE in these latter patients, and an informed decision should be made through a process of shared decision making incorporating patient values and preferences especially where the net clinical benefit of extended anticoagulation is more uncertain. Ideally, this assessment of the risk of bleeding is standardized and reproducible, that is, using a validated risk stratification scheme. For practical purposes, this scheme should be easy to assess and repeat (no complicated biomarkers), relevant to all currently available anticoagulant drug classes, specifically evaluated for patients with unprovoked VTE during chronic anticoagulant treatment, and not associated with a high risk of recurrent VTE: if patients at higher risk of bleeding are also at higher risk of recurrent VTE, the scheme is unlikely to be relevant for decision making, as was shown for the HAS-BLED score in AF.31 Finally, the scheme should at least consist of nonmodifiable risk factors.
Several bleeding risk prediction schemes have been developed and/or studied in patients with VTE (Tables 2 and 3).3,19,32-41 Most of these risk schemes have been derived and evaluated in studies with varying definitions of major bleeding and in patients with a broad range of VTE etiologies, including provoked VTE. More importantly, nearly all studies involved patients treated with warfarin, and the risk/benefit with warfarin may be distinctly different from the risk/benefit ratio with one of the DOACs, especially the FXa inhibitors. Variables of the different models largely overlap. Studies comparing the accuracy of these risk scores mostly report disappointing predictive accuracy with C-statistics in the range of 0.5 to 0.6, with little differences between the individual schemes. It should be noted that most of these studies were either retrospective or post hoc and did not involve independent adjudication of bleeding events.18,34,35,37,42-57 Moreover, only very few aimed specifically at the most relevant treatment period beyond the first 3 months. Of note, reporting measures of discrimination (ie, C-statistic) will always be important for a prediction model, but decision-analytic measures, such as absolute risks and risk differences between risk categories, are at least as relevant if the model is to be used for making clinical decisions: in most studies, the high-risk class of the different scores indeed involved a clearly higher risk than the low-risk class, even despite poor calibration statistics, indicating at least some clinical usefulness.58
Most relevant risk stratification schemes derived in different settings than VTE
| . | HAS-BLED38 . | mOBRI39 . | ATRIA78 . | HEMORR2HAGES40 . |
|---|---|---|---|---|
| Risk factors | ||||
| Age ≥64 y | 1 point | |||
| Age >65 y | 1 point | |||
| Age >75 y | 2 points | 1 point | ||
| Previous bleeding | 1 point | 1 point | 2 points | |
| Previous gastrointestinal bleeding | 1 point | |||
| Hepatic or renal disease | 1 point | |||
| Renal failure/insufficiency | 1 point | 3 points | ||
| Liver failure | 1 point | |||
| Previous stroke | 1 point | 1 point | 1 point | |
| Anemia | 3 points | 1 point | ||
| Antiplatelet therapy | 1 point | |||
| Labile international normalized ratio | 1 point | |||
| CYP2C9 single-nucleotide polymorphisms | 1 point | |||
| Excessive fall risk | 1 point | |||
| Reduced platelet count or function | 1 point | |||
| History of hypertension | 1 point | 1 point | 1 point | |
| Recent myocardial infarction, renal insufficiency, diabetes, or anemia | 1 point | |||
| Drugs/alcohol use | 1 point | |||
| Risk stratification | ||||
| Low risk | 0 points | 0 points | 0 to 3 points | 0 to 1 points |
| Intermediate risk | 1 to 2 points | 1 to 2 points | 4 points | 2 to 3 points |
| High risk | >2 points | >2 points | >4 points | >3 points |
| . | HAS-BLED38 . | mOBRI39 . | ATRIA78 . | HEMORR2HAGES40 . |
|---|---|---|---|---|
| Risk factors | ||||
| Age ≥64 y | 1 point | |||
| Age >65 y | 1 point | |||
| Age >75 y | 2 points | 1 point | ||
| Previous bleeding | 1 point | 1 point | 2 points | |
| Previous gastrointestinal bleeding | 1 point | |||
| Hepatic or renal disease | 1 point | |||
| Renal failure/insufficiency | 1 point | 3 points | ||
| Liver failure | 1 point | |||
| Previous stroke | 1 point | 1 point | 1 point | |
| Anemia | 3 points | 1 point | ||
| Antiplatelet therapy | 1 point | |||
| Labile international normalized ratio | 1 point | |||
| CYP2C9 single-nucleotide polymorphisms | 1 point | |||
| Excessive fall risk | 1 point | |||
| Reduced platelet count or function | 1 point | |||
| History of hypertension | 1 point | 1 point | 1 point | |
| Recent myocardial infarction, renal insufficiency, diabetes, or anemia | 1 point | |||
| Drugs/alcohol use | 1 point | |||
| Risk stratification | ||||
| Low risk | 0 points | 0 points | 0 to 3 points | 0 to 1 points |
| Intermediate risk | 1 to 2 points | 1 to 2 points | 4 points | 2 to 3 points |
| High risk | >2 points | >2 points | >4 points | >3 points |
Most widely evaluated risk prediction schemes
The RIETE bleeding score was published in 2008 (Table 2).33 Based on 314 bleeding events in >13 000 patients from the RIETE registry treated with VKA, 6 independent predictors were combined in a scheme that categorized patients in 3 categories of increasing risk. Several studies have since then evaluated the score.18,35,42,44-48 Main findings have been increasing incidences in the different risk categories but modest overall predictive value, especially on the long term. The score was not specifically tested in patients treated with dabigatran nor in patients with unprovoked VTE (Table 4).
Current status of best-studied risk stratification schemes for major bleeding in patients with VTE
| . | ACCP risk table3 . | VTE-BLEED19 . | RIETE33 . | HAS-BLED38 . |
|---|---|---|---|---|
| External evaluation in retrospective study | ✓ | ✓ | ✓ | ✓ |
| External evaluation in prospective study | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with VKA | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with DOACs | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with direct Xa inhibitors | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with direct thrombin inhibitors | ✓ | |||
| Association with risk of recurrent VTE established | ✓ | |||
| Association with risk of fatal/intracranial bleeding established | ✓ | |||
| Performance during long-term treatment established | ✓ | ✓ | ✓ | |
| Performance in patients with unprovoked VTE established | ✓ | |||
| Prospective validation in outcome study |
| . | ACCP risk table3 . | VTE-BLEED19 . | RIETE33 . | HAS-BLED38 . |
|---|---|---|---|---|
| External evaluation in retrospective study | ✓ | ✓ | ✓ | ✓ |
| External evaluation in prospective study | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with VKA | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with DOACs | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with direct Xa inhibitors | ✓ | ✓ | ✓ | ✓ |
| Evaluated in patients treated with direct thrombin inhibitors | ✓ | |||
| Association with risk of recurrent VTE established | ✓ | |||
| Association with risk of fatal/intracranial bleeding established | ✓ | |||
| Performance during long-term treatment established | ✓ | ✓ | ✓ | |
| Performance in patients with unprovoked VTE established | ✓ | |||
| Prospective validation in outcome study |
The 2016 clinical practice guideline from the ACCP suggested using a table, comprising 18 risk factors for major bleeding, in the decision for extended anticoagulant treatment (Table 2).3 Risk factors were selected after a review of the literature rather than by formal modeling of prospectively collected data. Because of that, the authors were forced to depend on the sometimes unclear definitions of risk factors in previous studies and could not weigh the relative impact of the individual risk factors. Instead, it was judged that patients with no risk factors would have a low risk of major bleeding, those with 1 risk factor would have a somewhat higher risk (moderate risk), and those with multiple risk factors would have a substantially higher risk (high risk). It was suggested that the ACCP bleeding risk tool could be used to consider anticoagulant withdrawal in patients at high risk of bleeding.3 Indeed, external validation studies have shown that the relative or proportional increase in the incidence of major bleeding moving from low to moderate to high categories were similar to what was estimated.49 As with the RIETE score, the overall predictive accuracy was found to be moderate.18,37,48,50
VTE-BLEED is a 6-variable scheme that was derived from the RE-COVER trials.19,59,60 All major and clinically relevant non–major bleeding events occurring in the patients randomized to treatment with dabigatran were used to identify single-risk factor or risk factor combinations. The predefined main outcome of the study was the predictive value of the combined risk factors for major bleeding after day 30 for the 2 treatment arms separately, as proxy for long-term treatment. VTE-BLEED proved to be a strong predictor for major bleeding during stable anticoagulation (odds ratio 7.5) with either dabigatran or warfarin. Its predictive strength was confirmed in the HOKUSAI and Xalia study, where patients were treated with Xa inhibitors and endpoints were adjudicated in an identical way as was performed in the RE-COVER trials.19,61,62 Although one of the VTE-BLEED variables is cancer, subgroup analyses in all 3 studies found adequate predictive value in patients with unprovoked non–cancer-associated VTE as well (three- to fivefold higher incidence of major bleeding in patients categorized as VTE-BLEED high risk than in those classified as low risk).19,61,62 Additional studies confirmed that VTE-BLEED was also predictive of fatal and intracranial bleeding events, but not of recurrent VTE.63,64 Recent studies comparing several risk-stratification schemes concluded that VTE-BLEED had good predictive strength relative to other schemes tested.51-54,57
HAS-BLED is probably the best validated scheme of all. It was derived from the Euro Heart Survey on AF and provided a practical tool to assess the individual bleeding risk of real-world patients with AF.38 In the AF population, HAS-BLED has been extensively validated in large cohorts and many important patient subgroups.31,65 Notably, the scheme involves the item “labile INR (international normalized ratio),” which is not relevant for DOAC treatment and lacks the item “cancer,” which is one of the strongest determinants of major bleeding in patients with VTE. Therefore, the scheme was modified in several of the validations studies performed in the setting of VTE, which consistently showed a low risk of major bleeding in the HAS-BLED low-risk category.5,18,19,48,54-56
Current international AF guidelines advocate application of bleeding risk scores only to identify modifiable risk factors but not to withhold treatment in patients at high risk of bleeding because of the net clinical benefit of oral anticoagulation across all bleeding risk categories.31 Because none of the available risk schemes has been tested in a formal VTE outcome study, and considering experiences from AF, bleeding risk stratification schemes should not (yet) be used in VTE patients as a main argument to discontinue anticoagulant treatment after 3 months.31,65,66 Risk stratification schemes may currently be used to identify patients at low risk of bleeding in whom the safety of indefinite treatment can be affirmed, with the ACCP risk table and VTE-BLEED being the best validated tools available.
Long-term follow-up
In patients who receive extended anticoagulation, it is recommended that their drug tolerance and adherence, hepatic and renal function, and bleeding risk are reassessed at regular intervals (Figure 1).4,8 Specifically, the presence of new modifiable risk factors for bleeding should be checked at least once a year, whereas the evolution of established risk factors, such as hypertension or renal insufficiency, should be checked more frequently, depending on their severity. Patients with unprovoked VTE and 1 or more risk factors for bleeding could receive the reduced doses of either apixaban (2.5 mg twice daily) or rivaroxaban (10 mg once daily), although it remains to be studied whether treatment with reduced dosed DOAC is associated with a lower risk of major bleeding than treatment with a standard dosed DOAC. Risk stratification schemes can also be used to monitor changes in bleeding risk profile over time. The benefit of their longitudinal assessment rather than application as stand-alone baseline assessments should be studied, to help determine the appropriateness of treatment regimens reflective of new or worsening risk factors.
The art of prediction
Over the past decade, many studies have focused on risk of bleeding in patients with VTE. To translate that knowledge into clinical practice, the reason for the prediction and moment in the course of disease should match those of the derivation and validation studies. It is important to differentiate between modifiable and nonmodifiable risk factors: although modifiable risk factors are relevant throughout the complete course of anticoagulant treatment, nonmodifiable risk factors are mostly relevant for long-term management decisions. Furthermore, the art of prediction involves the combination of common clinical sense and evidence-based medicine. Even without level 1a evidence, we should pursue reversing modifiable risk factors where possible, whereas, in contrast, we cannot readily withhold long-term anticoagulant treatment only based on a single assessment of a risk stratification scheme. Future studies should determine the definitive role of the stratification schemes, and in particular, the ACCP tool and VTE-BLEED, and integrate prediction models for major bleeding and recurrent VTE to establish the net clinical benefit of specific management strategies in different clinical settings. Importantly, the measurement of the net clinical benefit should not only involve combining the absolute risks of recurrent VTE and major bleeding but also weigh the impact of such events, that is, the case fatality rate and impact on functionality in daily life of the survivors of such complications.28-30,67
Case scenarios: resolution
Scenario 1
The patient was diagnosed with DVT shortly after a neurosurgical procedure. We identified 1 modifiable risk factor for bleeding, that is, use of aspirin, and 1 notable nonmodifiable risk factor, the recent surgery. Because 3 days had passed since this particular type of surgery, anticoagulation therapy was not considered absolutely contraindicated, and treatment with twice-daily dosed anticoagulation LMWH was initiated; aspirin was discontinued. Of note, the timeframe between the neurosurgical procedure and the “safe” initiation of anticoagulant treatment may differ and should be determined at the individual level. We initially did not prescribe a DOAC, because apixaban and rivaroxaban require an initial starting dose for 1 week and 3 weeks, respectively, that is 50% to 100% higher than the normal dose and may increase the risk of bleeding compared with LMWH in a standard dose in the setting of our patient. After 3 days, the patient suffered from a nosebleed that was treated with tranexamic acid and a hemostatic nasal tampon. The subsequent dose of LMWH was skipped, and the next administration was decreased to a prophylactic dose. Full-dose anticoagulation was restarted 24 hours after the bleed, and no further bleeding complications occurred. Upon discharge, his blood pressure and hemoglobin levels were normal. He was switched to a direct oral Xa inhibitor and was discharged. Because of the clear provoking factor for the VTE indicating low risk of recurrence, anticoagulant treatment was discontinued after 3 months and the aspirin was restarted. Of note, aspirin has been shown to be associated with a modest decrease in the risk of recurrent VTE as compared with placebo, but this is not relevant for patients with VTE provoked by a major transient risk factor.4
Scenario 2
Several considerations are relevant to this case. In the absence of Hestia criteria, she may be treated at home.68 In such cases, apixaban and rivaroxaban may be preferred because initial treatment with LMWH is then avoided. However, direct Xa inhibitors are associated with a higher risk of abnormal menstrual bleeding.69-74 Dabigatran may be associated with a lower risk of abnormal uterine bleeding but is not the ideal drug in patients with renal insufficiency, and its start must be preceded by a short course of LMWH.75 Of note, the RECOVER trials were not a priori designed to study the effect of dabigatran on menstrual bleeding. Last, she has 2 potential modifiable risk factors for bleeding, that is, hypertension and renal insufficiency. After discussing the risk of abnormal menstrual bleeding with her, treatment with an oral Xa inhibitor was started, and she was discharged home. One week after initial presentation, she visited the outpatient clinic where renal function, blood pressure, and medication adherence were checked. Because of a blood pressure of 142/85 measured in the office and later also ambulatory, the dose of the calcium channel blocker was increased with an adequate effect on the blood pressure. After 6 weeks, she reported relevant menorrhagia and had a notable new anemia. She was switched to dabigatran 150 mg twice daily, after confirming stable renal function. Furthermore, she was counseled to receive an intrauterine device with progestogen.71,76 At the 3 months follow-up visit, the menstruation had turned normal. Her renal function was stable; her hypertension was very well managed, and the anemia resolved. She was considered to be at low risk of bleeding according to VTE-BLEED, and it was discussed with the patient that she was currently at low risk of major bleeding. She expressed a clear preference to continue anticoagulant treatment to maximally reduce the risk of recurrent VTE. We agreed and planned regular evaluations of diabetes regulation, renal function, blood pressure, and health status 3 times a year. A future decline of renal function or incident major bleeding episode especially should trigger reevaluation of the current anticoagulant regimen.
Conclusion
Even in the absence of fully established risk prediction schemes and randomized trials proving the absolute benefit of treatment decisions based on standardized bleeding prediction, prediction of major bleeding in patients with VTE is relevant from start to discontinuation of anticoagulant treatment. Managing the risk of bleeding involves more than application of bleeding prediction schemes: the present evidence supports careful selection of the optimal anticoagulant drug and correct dosing, screening for and targeting of modifiable risk factors for major bleeding as well as the application of decision rules to identify patients at low risk of bleeding complications, in whom long-term anticoagulant treatment is likely safe.
Authorship
Contribution: F.A.K. drafted the manuscript; M.V.H. critically revised the draft for important intellectual content; and both authors approved the final version of the manuscript.
Conflict-of-interest disclosure: F.A.K. reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch Thrombosis Association, and the Dutch Heart Foundation. M.V.H. reports receiving research grants from ZonMW, Boehringer Ingelheim, Bayer Health Care, and Pfizer-Bristol-Myers Squibb and has received consultancy and lecture fees from Pfizer-Bristol-Myers Squibb, Boehringer Ingelheim, Bayer Health Care, and Aspen.
Correspondence: Frederikus A. Klok, Department of Medicine–Thrombosis and Hemostasis, Leiden University Medical Center, Albinusdreef 2, Leiden, 2300 RC, The Netherlands; e-mail: f.a.klok@LUMC.nl.