TO THE EDITOR:
Asparaginase is a critical component of acute lymphoblastic leukemia (ALL) therapy. Available asparaginase products in the United States include pegaspargase (Oncaspar), asparaginase Erwinia chrysanthemi (Erwinaze), and calaspargase (Asparlas), with pegaspargase used more commonly in first-line treatment. Allergic reactions to pegaspargase occur in 10% to 15% of patients,1-3 a rate that may be decreased to 4% to 5% using premedications during initial therapy.4,5
Options for patients after allergic reactions to pegaspargase include rechallenging with premedications, switching to Erwinia asparaginase, or discontinuation of asparaginase therapy.6 Marini et al.7 developed a multidisciplinary committee to review pegaspargase hypersensitivity reactions and followed an algorithm for premedications and 2-hour infusion with a reported decrease in Erwinia asparaginase use from 21% to 7%. However, as this and other data indicate, conventional premedication and infusion modifications still fail in a significant number of patients with prior allergic reactions, highlighting the need for a new asparaginase treatment strategy. Failure to adequately deplete asparagine has been associated with decreased event-free survival and increased risk for central nervous system relapse.8-10 Drug substitutions during drug shortages have previously been shown to result in inferior oncology therapy outcomes.11 Because of inconsistent Erwinia asparaginase availability, we searched for alternative methods of administering pegaspargase after hypersensitivity reaction.
Rapid drug desensitization protocols have been used after hypersensitivity reaction for other chemotherapy agents including carboplatin, taxanes, and monoclonal antibodies.12 Limited experience with pegaspargase desensitization has been reported,13-15 and these data have been limited by incomplete evaluations of anti-pegaspargase antibodies and activity, factors known to influence the efficacy of therapy.8-10 We previously identified polyethylene glycol rather than l-asparaginase as the primary antigen in pegaspargase allergic reactions.3 Anti-l-asparaginase antibodies more negatively affected asparaginase activity than anti-polyethylene glycol antibodies, suggesting that patients tolerating pegaspargase desensitization may be able to maintain adequate asparaginase activity levels.3
Because of the ongoing national shortage of Erwinia asparaginase and the need to provide effective asparagine depletion for maximal effective anti-leukemic therapy, we evaluated a rapid desensitization protocol in patients with a history of severe pegaspargase allergic reaction and monitored antibodies and asparaginase activity to assess the efficacy of the desensitization.
We retrospectively reviewed all patients desensitized to pegaspargase at our institution from January 1 to June 17, 2019. Patients/guardians provided informed consent and patient assent consistent with the Declaration of Helsinki. All research was approved by the institutional review board.
A 12-step desensitization procedure was used (supplemental Data, available on the Blood Web site). Pegaspargase was diluted with normal saline in 3 bags (0.05 units/mL, 0.5 units/mL, and 10 units/mL). Infusions used 4 steps per bag titrated every 15 minutes to a maximum rate of 120 mL/hour for step 12. Steps 1 to 11 of the infusion were the same for all patients. Step 12 was calculated on the basis of the patient’s body surface area. Premedications consisting of diphenhydramine, hydrocortisone, ranitidine, and montelukast were given before desensitization and continued for at least 24 hours. All patients were monitored in the intensive care unit for their initial desensitized dose. Dose administration was terminated early if patients developed facial edema or cardiopulmonary symptoms, or at the physician’s discretion. Subsequent pegaspargase doses were also given via the desensitization protocol if patients tolerated the first dose and maintained asparaginase activity higher than 0.1 IU/mL for at least 14 days.
Antibodies were collected on all patients before desensitization. Asparaginase activity was obtained at 2 to 4 points after desensitization: infusion completion, 24 to 96 hours, 7 days, and 14 days after desensitization. Antibodies and asparaginase activity were determined according to methods previously described.3
Patient characteristics of the 8 patients desensitized are described in Table 1. Median time from initial pegaspargase reaction to desensitization for 7 patients with documented reaction dates was 143 days (range, 4-224 days). Seven patients (87.5%) were able to complete the infusion. The only adverse effect observed during desensitization was rash, occurring in 3 patients (37.5%). Infusion was stopped early in 1 patient because of progression of rash despite additional hydrocortisone and diphenhydramine. The longest infusion time was 12 hours as a result of recurrence of rash and inability to exceed an infusion rate of 30 mL/hour (10 unit/mL concentration). Four patients received subsequent doses during the study period without complications. Six patients (75%) were able to maintain asparaginase activity higher than 0.1 IU/mL for at least 2 weeks.
Patient characteristics
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Age, y | 8 | 19 | 8 | 4 | 16 | 2 | 4 | 3 |
| Sex | M | M | F | M | M | F | F | F |
| Immunophenotype/protocol risk | T-ALL HR | ETP-ALL HR | B-ALL SR/HR | B-ALL LR | B-ALL SR/HR | Rel KMT2Ar B-ALL HR | B-ALL LR | B-ALL SR/HR |
| Symptoms of initial PEGASP reaction | Emesis, itching eyes, chest pain, hypotension, lip swelling, flushing requiring ICU admission | Emesis, oxygen desaturation, rash | Hypotension, oxygen desaturation, facial and throat swelling, hives | Cough, rash on face, emesis, lip and eye swelling | Throat and facial tightness, nausea (no emesis) | Lip, tongue and eye swelling, itching, hives | Emesis, swollen eyes and lips | Face redness, emesis and lip swelling, hypotension requiring ICU admission |
| Initial reaction CTCAE grade | 4 | 3 | 4 | 3 | 3 | 2 | 3 | 4 |
| Premedication at initial reaction | HC | HC, Ran, Diphen | Unknown | HC, Ran, Diphen | HC, Ran, Diphen | Unknown | HC, Ran, Diphen | HC, Ran, Diphen |
| Antibodies around time of initial reaction | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze−(2.5 mo after initial reaction) | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP+, Erwinaze− | Unknown | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze− |
| Anti-PEG−ASP OD: 0.1405 | PEG−ASP OD: 0.6110 | PEG−ASP OD: 0.3675 | PEG−ASP OD: 0.3487 | PEG−ASP OD: 0.2843 | PEG−ASP OD: 0.2584 | PEG−ASP OD: 0.3365 | ||
| PEG OD: 0.4030 | ||||||||
| Time from initial reaction to desensitization, days | 150 | 119 | 224 | 4 | 193 | Unknown | 143 | 90 |
| Interval Erwinaze between reaction and desensitization | Yes | Yes | Yes | No | Yes | Unknown | Yes | No |
| Presence of antibodies before desensitization | PEG−ASP −, PEG NA, Erwinaze− | PEG−ASP+, PEG+, Erwinaze+ | PEG−ASP−, PEG−, Erwinaze− | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP−, PEG−, Erwinaze− | PEG−ASP+, PEG+, Erwinaze+ | PEG−ASP−PEG−, Erwinaze− |
| PEG-ASP OD: 0.0345 | PEG-ASP OD: 0.6546 | PEG-ASP OD: 0.0855 | PEG-ASP OD: 0.3160 | PEG-ASP OD: 0.122 | PEG-ASP OD: 0.0099 | PEG-ASP OD: 0.6374 | PEG-ASP OD: 0.0579 | |
| PEG OD: 0.757 | PEG OD: 0.1005 | PEG OD: 0.4375 | PEG OD: 0.188 | PEG OD: 0.0260 | PEG OD: 0.6880 | PEG OD: 0.098 | ||
| Number of desensitized doses received | 7 | 1 | 4 | 1 | 3 | 2 | 1 | 1 |
| Symptoms during desensitization | None | Rash at end of infusion but received planned dose | None | Rash × 3 during infusion. Received planned dose over 12 hours | None | None | Rash during infusion, unable to complete infusion; infused 916 units/m2 | None |
| Reason dose not repeated | N/A | Thrombosis, progressive leukemia | N/A | Unable to maintain activity >0.1 for a week | N/A | N/A | Did not tolerate infusion | N/A |
| Estimated day 14 activity after 1st desensitized dose ≥0.1 IU/mL | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Age, y | 8 | 19 | 8 | 4 | 16 | 2 | 4 | 3 |
| Sex | M | M | F | M | M | F | F | F |
| Immunophenotype/protocol risk | T-ALL HR | ETP-ALL HR | B-ALL SR/HR | B-ALL LR | B-ALL SR/HR | Rel KMT2Ar B-ALL HR | B-ALL LR | B-ALL SR/HR |
| Symptoms of initial PEGASP reaction | Emesis, itching eyes, chest pain, hypotension, lip swelling, flushing requiring ICU admission | Emesis, oxygen desaturation, rash | Hypotension, oxygen desaturation, facial and throat swelling, hives | Cough, rash on face, emesis, lip and eye swelling | Throat and facial tightness, nausea (no emesis) | Lip, tongue and eye swelling, itching, hives | Emesis, swollen eyes and lips | Face redness, emesis and lip swelling, hypotension requiring ICU admission |
| Initial reaction CTCAE grade | 4 | 3 | 4 | 3 | 3 | 2 | 3 | 4 |
| Premedication at initial reaction | HC | HC, Ran, Diphen | Unknown | HC, Ran, Diphen | HC, Ran, Diphen | Unknown | HC, Ran, Diphen | HC, Ran, Diphen |
| Antibodies around time of initial reaction | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze−(2.5 mo after initial reaction) | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP+, Erwinaze− | Unknown | PEG−ASP+, Erwinaze− | PEG−ASP+, Erwinaze− |
| Anti-PEG−ASP OD: 0.1405 | PEG−ASP OD: 0.6110 | PEG−ASP OD: 0.3675 | PEG−ASP OD: 0.3487 | PEG−ASP OD: 0.2843 | PEG−ASP OD: 0.2584 | PEG−ASP OD: 0.3365 | ||
| PEG OD: 0.4030 | ||||||||
| Time from initial reaction to desensitization, days | 150 | 119 | 224 | 4 | 193 | Unknown | 143 | 90 |
| Interval Erwinaze between reaction and desensitization | Yes | Yes | Yes | No | Yes | Unknown | Yes | No |
| Presence of antibodies before desensitization | PEG−ASP −, PEG NA, Erwinaze− | PEG−ASP+, PEG+, Erwinaze+ | PEG−ASP−, PEG−, Erwinaze− | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP+, PEG+, Erwinaze− | PEG−ASP−, PEG−, Erwinaze− | PEG−ASP+, PEG+, Erwinaze+ | PEG−ASP−PEG−, Erwinaze− |
| PEG-ASP OD: 0.0345 | PEG-ASP OD: 0.6546 | PEG-ASP OD: 0.0855 | PEG-ASP OD: 0.3160 | PEG-ASP OD: 0.122 | PEG-ASP OD: 0.0099 | PEG-ASP OD: 0.6374 | PEG-ASP OD: 0.0579 | |
| PEG OD: 0.757 | PEG OD: 0.1005 | PEG OD: 0.4375 | PEG OD: 0.188 | PEG OD: 0.0260 | PEG OD: 0.6880 | PEG OD: 0.098 | ||
| Number of desensitized doses received | 7 | 1 | 4 | 1 | 3 | 2 | 1 | 1 |
| Symptoms during desensitization | None | Rash at end of infusion but received planned dose | None | Rash × 3 during infusion. Received planned dose over 12 hours | None | None | Rash during infusion, unable to complete infusion; infused 916 units/m2 | None |
| Reason dose not repeated | N/A | Thrombosis, progressive leukemia | N/A | Unable to maintain activity >0.1 for a week | N/A | N/A | Did not tolerate infusion | N/A |
| Estimated day 14 activity after 1st desensitized dose ≥0.1 IU/mL | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
Underlined symptoms are those associated with desensitization failure when combined with persistent anti-PEG asparaginase antibodies.
CTCAE, common terminology criteria for adverse events, version 3, allergic reaction; Diphen, diphenhydramine; HC, hydrocortisone; HR, high-risk; ICU, intensive care unit; LR, low risk; N/A, not applicable; OD, optical density; OSH, outside hospital; PEG, polyethylene glycol; PEG-ASP, pegaspargase; Ran, ranitidine; Rel, relapsed; SR, standard risk.
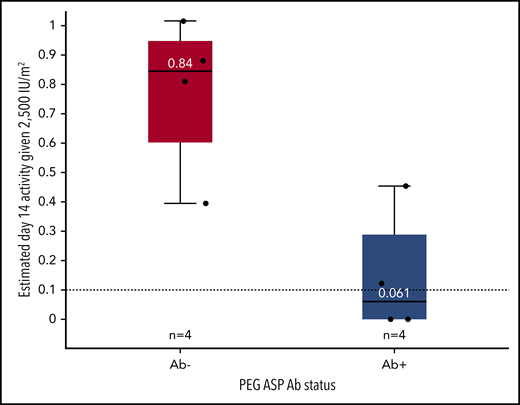
Seven patients had documented antibodies to pegaspargase when they initially reacted (1 patient had no data available and was negative at the time of desensitization). Before desensitization, 3 patients had become antibody negative. All 4 patients without antibodies to polyethylene glycol (PEG) or pegaspargase (PEG-ASP) were successfully desensitized and able to maintain asparaginase activity higher than 0.1 IU/mL for at least 2 weeks. Two of 4 patients positive for antibodies to PEG and PEG-ASP had reactions during desensitization and were unable to maintain adequate asparaginase activity. Patients without antibodies at the time of pegaspargase desensitization had higher average 14-day activity than patients with antibodies (0.84 vs 0.061 IU/mL; P = .043; Figure 1). Results of antibody testing in 4 patients after desensitization are shown in the supplement.
Antibody status influences day 14 asparaginase activity after desensitization. Estimated day 14 activity based on a 2500 IU/m2 dose of PEG-ASP subdivided by antibody status before desensitization. The boxes represent the median and interquartile range, and the whiskers represent the range. Individual patient activity estimates are indicated with dots.
Antibody status influences day 14 asparaginase activity after desensitization. Estimated day 14 activity based on a 2500 IU/m2 dose of PEG-ASP subdivided by antibody status before desensitization. The boxes represent the median and interquartile range, and the whiskers represent the range. Individual patient activity estimates are indicated with dots.
Facial angioedema occurred in 6 patients at the time of initial pegaspargase reaction, and 4 patients (66.7%) were successfully desensitized. The other 2 patients had antibodies to pegaspargase before desensitization and failed desensitization. Characteristics of the initial pegaspargase reaction or antibody status alone were not indicative of ability to tolerate desensitization.
These data suggest that asparaginase antibody and activity monitoring and symptoms of previous hypersensitivity reaction are important when desensitizing patients with pegaspargase. Patients with antibodies who are desensitized are at risk for rapid pegaspargase clearance and may have inadequate asparaginase activity after desensitization. Our data also report on the first cohort of patients rechallenged with pegaspargase after previously documented antibody-positive reactions who subsequently converted to antibody negative. It is unclear why these 3 patients converted to antibody negative, and studies of larger cohorts are needed to identify factors that predict conversion from antibody positive to negative. These patients all tolerated desensitization well, and future studies may consider rechallenge without desensitization in this population.
In prior reports, 7 of 10 patients who completed pegaspargase desensitization infusion maintained asparaginase activity of at least 0.1 IU/mL for at least 10 days.15 We report similar success rates, with 7 patients completing the infusion and 6 maintaining asparaginase activity of at least 0.1 IU/mL for at least 14 days. Notably, only 1 of 3 patients with symptoms of hypersensitivity during desensitization despite premedications and slow infusion was able to maintain activity.
In patients rechallenged with pegaspargase without desensitization, angioedema, and gastrointestinal symptoms during the initial reaction, as well as pre-rechallenge antibody positivity, were associated with rechallenge failure.3 The 2 patients who failed desensitization in our cohort had the triad of angioedema, emesis, and ongoing positive antibodies prior to the time of desensitization. Caution should be used when desensitizing patients with persistent antibodies and prior history of angioedema with gastrointestinal symptoms, as they are at a high risk for rechallenge failure either because of symptoms during their infusion or rapid drug clearance/inactivation.
Our study confirms the feasibility of drug desensitization in patients with persistent anti-pegaspargase antibodies. It also demonstrates the importance of monitoring antibodies to pegaspargase and asparaginase activity and documentation of symptoms in the previous hypersensitivity reaction in this population. Further studies are needed to understand the predictors of asparaginase activity and antibody response after desensitization to pegaspargase.
Acknowledgments
The authors thank May Chung, Jean Cai, and Pam McGill for analytical work.
M.V.R. reports receiving investigator-initiated research funding from Servier. Additional support was received from American Lebanese Syrian Associated Charities, and from National Institutes of Health, National Cancer Institute grant CA21765.
Authorship
Contribution: H.D.S. and S.E.K. designed the study, analyzed data, and performed analyses; J.C.P. analyzed data and performed analyses; H.I. and C.-H.P. contributed patients and are principle investigators of the Total 17 trial; and all authors contributed to manuscript preparation and revision.
Correspondence: Seth E. Karol, St. Jude Children's Research Hospital, 262 Danny Thomas Pl, Mail Stop 260, Memphis, TN 38105; e-mail: seth.karol@stjude.org.
REFERENCES
Author notes
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal