Background: Acute myeloid leukemia (AML) outcomes in the elderly, particularly intensive chemotherapy (IC)-ineligible patients (pts), are poor. Venetoclax (VEN), a BCL2 inhibitor, in combination with low-intensity regimens has shown excellent efficacy in AML and is approved for IC-ineligible pts as frontline therapy. Outcomes and expectations of AML after failure of frontline VEN-based regimens are unknown.
Methods: We conducted a retrospective study to determine pt outcomes after failure of frontline therapy with VEN and hypomethylating agents (HMA). Newly diagnosed (ND) AML pts enrolled on 2 clinical trials of VEN+HMA (NCT02203773, NCT03404193) with refractory AML or relapse after initial response to VEN+HMA were included. In 1 trial, ND IC-ineligible AML pts (≥65 years [yr]) received VEN 400-1200 mg daily with decitabine (DEC) for 5 days or azacitidine (AZA) for 7 days. The other trial enrolled ND IC-ineligible AML pts (>60 yrs) who received VEN 400 mg daily or equivalent with DEC for 10 days until CR/CRi, followed by 5-day cycles. FLT3 inhibitors (FLT3i) were allowed in FLT3mut pts. Overall survival (OS) was measured from date of diagnosis of refractory AML or relapse after VEN+HMA therapy, till death or censored at last follow-up. The data cut-off date was 07.08.19.
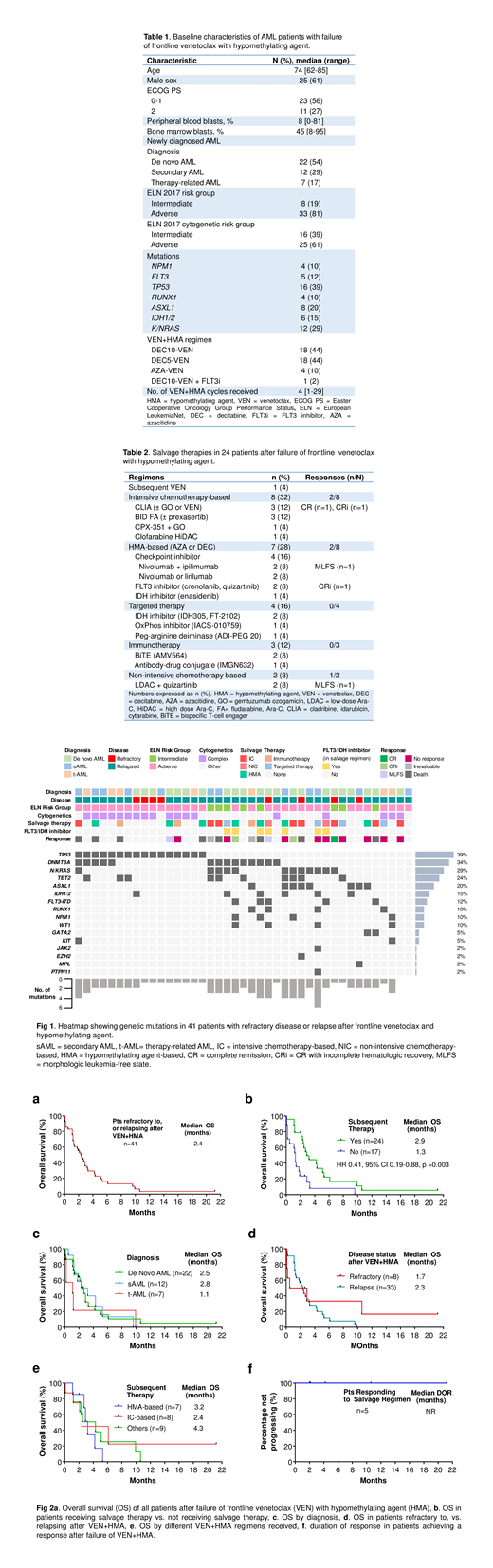
Results: Between November 2014 and February 2019, out of 103 ND AML pts treated with VEN+HMA, 41 pts were identified to have refractory AML, or relapse after VEN+HMA. The median age was 74 yrs (range 62-85), 12 pts (29%) had sAML, 7 pts (17%) had therapy-related AML, 33 pts (81%) had ELN adverse risk AML, 16 pts (39%) had TP53mut, 12 pts (29%) had N/KRASmut, and 5 pts (12%) had FLT3-ITD (Table 1, Fig 1). Pts had received a median of 4 cycles of VEN+HMA (range 1-29). The median follow-up duration for all pts was 21.2 months (mo).
With frontline VEN+ HMA, 19 pts (46%) achieved CR, 11 pts (27%) achieved CRi, 3 pts (7%) achieved morphologic leukemia free state (MLFS), and 8 pts (20%) had primary refractory disease. Pts obtaining initial response relapsed after a median duration of response (DOR) of 5.3 mo (range 0.9-34.1). After VEN+ HMA failure, median OS for all 41 pts was 2.4 mo (range 0.1-21.2, Fig 2a). Pts who received salvage therapy (n=24) had longer OS compared to pts who did not receive salvage therapy (n=17, 2.9 vs 1.3 mo, HR=0.41, 95% CI 0.19-0.88, p=0.003, Fig 2b). Median OS for de novo AML at relapse/failure was 2.5 mo, for sAML was 2.8 mo, and for t-AML was 1.1 mo (Fig 2c). Pts with primary refractory AML vs relapsed AML had comparable OS of 1.7 mo vs 2.3 mo, respectively (Fig 2d).
Of the 24 pts who received salvage therapy (Table 2, Fig 2e), 5 pts (21%) responded; CR in 1 pt, CRi in 2 pts and MLFS in 2 pts. Among 3 pts with primary refractory AML, 1 pt achieved CR and 1 pt achieved MLFS. Among 21 pts with relapse after VEN+HMA, 2 pts achieved CRi and 1 pt achieved MLFS. 8 pts received IC, and 2/8 pts (notably both with NRASmut) achieved CR and CRi with CLIA, and CLIA + gemtuzumab ozogamicin, respectively. 7 pts received HMA-based regimens, and 2/7 pts responded with CRi and MLFS with AZA + quizartinib, and AZA + nivolumab + ipilimumab, respectively. The former pt had FLT3-ITD and NRASmut and the latter pt had TP53mut. Of the remaining 9 pts receiving other therapies, 1 pt with FLT3-ITD achieved a MLFS with quizartinib + low-dose ara-c. These 5 responding pts continue in remission with median DOR not reached (NR, range 0.7-20.1, Fig 2f) and OS also NR (range, 2-21.2).
All pts with NPM1mut and IDH1/2mut who relapsed had adverse-risk cytogenetics or co-occurring mutations in TP53, N/KRAS, FLT3, and/or KIT. Of FLT3-ITDmut pts, 2 of 5 pts (40%) responded to salvage therapy including a FLT3i. Of 11 RASmut pts, 3 pts (27%) responded to salvage therapy including both IC and HMA-based regimens. 1 of 6 TP53mut pts receiving salvage therapy achieved MLFS with AZA + nivolumab + ipilimumab. This pt was also the only one among 7 pts with complex cytogenetics who responded to salvage therapy.
Conclusion: Older IC-ineligible pts with ND AML who fail frontline VEN+HMA have high-risk disease biology including t-AML, sAML, complex cytogenetics, FLT3-ITD, TP53mut, N/KRASmut. These known high-risk features decrease likelihood of response and confer poor OS, confirmed in this analysis. The median OS after frontline VEN+HMA failure was 2.4 mo. Notably some pts with FLT3-ITD responded well to salvage regimens with FLT3i. Novel therapies to abrogate VEN resistance, especially in high risk genotypes, are urgently needed.
Maiti:Celgene: Other: research funding. Cortes:Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding. Pemmaraju:mustangbio: Consultancy, Research Funding; abbvie: Consultancy, Honoraria, Research Funding; samus: Research Funding; celgene: Consultancy, Honoraria; cellectis: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Research Funding; plexxikon: Research Funding; Daiichi-Sankyo: Research Funding; sagerstrong: Research Funding; affymetrix: Research Funding; incyte: Consultancy, Research Funding. Daver:Immunogen: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Jazz: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Servier: Research Funding; Abbvie: Consultancy, Research Funding; Forty-Seven: Consultancy; Glycomimetics: Research Funding; Celgene: Consultancy; Astellas: Consultancy; Immunogen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy; Celgene: Consultancy; NOHLA: Research Funding; Agios: Consultancy; Otsuka: Consultancy; BMS: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Servier: Research Funding; Glycomimetics: Research Funding; Pfizer: Consultancy, Research Funding; NOHLA: Research Funding; Genentech: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; Agios: Consultancy; Karyopharm: Consultancy, Research Funding; Forty-Seven: Consultancy; Otsuka: Consultancy; Jazz: Consultancy; Incyte: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding. Ravandi:Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding; Menarini Ricerche: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Borthakur:AstraZeneca: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; NKarta: Consultancy; Cyclacel: Research Funding; Janssen: Research Funding; Incyte: Research Funding; Novartis: Research Funding; Xbiotech USA: Research Funding; Eisai: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Polaris: Research Funding; Arvinas: Research Funding; Merck: Research Funding; Cantargia AB: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Consultancy; Strategia Therapeutics: Research Funding; Bayer Healthcare AG: Research Funding; Agensys: Research Funding; Oncoceutics: Research Funding; GSK: Research Funding; BMS: Research Funding; Oncoceutics, Inc.: Research Funding; Eli Lilly and Co.: Research Funding; AbbVie: Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Celgene: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Jain:Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Andreeff:BiolineRx: Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; NIH/NCI: Research Funding; CPRIT: Research Funding; Breast Cancer Research Foundation: Research Funding; Oncolyze: Equity Ownership; Oncoceutics: Equity Ownership; Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership; Aptose: Equity Ownership; Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Amgen: Consultancy; AstaZeneca: Consultancy; 6 Dimensions Capital: Consultancy; Reata: Equity Ownership; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees. Bose:Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; CTI BioPharma: Research Funding. Jabbour:Takeda: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Thompson:AbbVie: Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Genentech: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Zhang:The University of Texas M.D.Anderson Cancer Center: Employment. Kantarjian:Agios: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Ariad: Research Funding; Cyclacel: Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Novartis: Research Funding; Takeda: Honoraria; Astex: Research Funding; BMS: Research Funding. Konopleva:Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding. DiNardo:jazz: Honoraria; abbvie: Consultancy, Honoraria; celgene: Consultancy, Honoraria; medimmune: Honoraria; syros: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; agios: Consultancy, Honoraria; daiichi sankyo: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal