
Background: Achievement of CR with full hematologic recovery and MRD negativity are independently associated with improved survival in pts with AML undergoing frontline treatment. However, in R/R AML, the prognostic impact of these response parameters is not well-established.
Methods: We retrospectively analyzed pts with R/R AML who received first salvage with an intermediate- or high-dose cytarabine regimen at our institution between 8/2011 and 7/2018. Only pts who achieved morphological remission, with or without full hematologic recovery, and had MRD measured at the time of best response were included in this analysis. Pts with core-binding factor AML were excluded. MRD was assessed by 8‐color multiparameter flow cytometry on bone marrow specimens with a sensitivity of 0.1% or higher. The primary outcomes were cumulative incidence of relapse (CIR), relapse-free survival (RFS) and overall survival (OS).
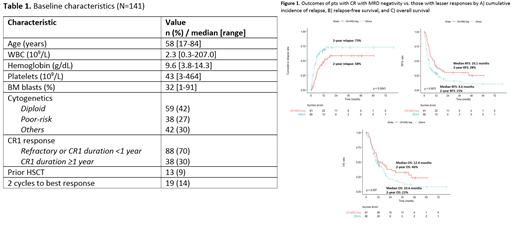
Results: A total of 141 pts met inclusion criteria and were included in the analysis. Table 1 shows the baseline characteristics of the study population.
Ninety-five pts (67%) achieved CR, 26 (18%) achieved CR with incomplete hematologic recovery (CRi), and 20 (14%) achieved morphological leukemia-free state (MLFS) as best response. Overall, 86 pts (61%) achieved MRD negativity. MRD negativity rates were similar among response groups (64%, 46%, and 65% for CR, CRi, and MLFS, respectively; P=0.22). The rate of CR was higher in pts with diploid vs. non-diploid karyotype (78% vs. 59%; P=0.02) and in pts with CR1 duration ≥1 year vs. others (87% vs. 57%; P=0.001). The MRD negativity rate was higher in pts with higher baseline bone marrow blasts (median blasts: 35% vs. 26%; P=0.03) and was not associated with any other pretreatment parameter.
The median duration of follow-up was 30.5 months. Pts who achieved CR vs. lesser response had lower CIR (P=0.01) and RFS (P=0.004) but not OS (P=0.15); a similar trend was observed in pts who achieved MRD negativity vs. those who were MRD-positive (P=0.01, P=0.05, and P=0.21, respectively). Among MRD-positive pts, level of MRD (i.e. <0.1% vs. 0.1%-1% vs. >1%) did not impact outcomes.
Overall, 62 pts (44%) underwent allogeneic HSCT after first salvage therapy, with a median time of 1.4 months between second remission and HSCT. HSCT after first salvage therapy was the strongest prognostic factor for CIR, RFS and OS (P<0.001 for all). HSCT rate was higher in those who achieved CR vs. lesser response (52% vs. 28%; P=0.008) or who were MRD-negative vs. MRD-positive (52% vs. 31%; P=0.01). The relapse rate within 1.4 months following second remission was high in pts who only achieved CRi/MLFS and/or were MRD-positive (16% vs. 3% for pts who achieved CR with MRD negativity; P=0.01) and was a major driver of the inferior outcomes in these groups.
In a landmark HSCT analysis, 22 pts who relapsed, died or lost to follow-up within 1.4 months of second remission were excluded. Among the remaining 57 pts who did not undergo HSCT in second remission, stratification of pts into 4 groups by hematologic response (CR vs. CRi/MLFS) and MRD response (positive vs. negative) was associated with significant difference in CIR (P=0.004) and RFS (P=0.008) but not OS (P=0.8). In contrast, among the 62 pts who underwent HSCT, neither hematologic nor MRD response was associated with CIR, RFS or OS. Similarly, MRD status immediately prior to HSCT was also not associated with outcomes.
By multivariate analysis including known predictors for outcomes in R/R AML (Breems DA et al. J Clin Oncol 2005) and using HSCT as a time-dependent variable, both CR and MRD negativity were independently associated with lower CIR (P=0.001 and P=0.003, respectively) and better RFS (P<0.001 and P=0.02, respectively) but not with OS. Overall, pts who achieved CR with MRD negativity (n=61) had the best outcomes (2-year CIR: 58% vs. 73%, P=0.004; 2-year RFS: 28% vs. 15%, P=0.008; 2-year OS: 46% vs. 21%, P=0.10; Figure 1A-C).
Conclusions: In pts with R/R AML receiving first salvage therapy, CR and achievement of MRD negativity were both independently associated with decreased CIR and longer RFS. The impact of hematologic recovery and MRD response in the salvage setting was largely limited to pts who did not undergo subsequent HSCT, whereas outcomes were similar for those who received HSCT. In the R/R setting, pts who achieve both CR and MRD negativity have the best outcomes, supporting CR with MRD negativity as the optimal response to salvage therapy.
Short:Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria. Daver:Servier: Research Funding; Otsuka: Consultancy; Sunesis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Forty-Seven: Consultancy; Karyopharm: Consultancy, Research Funding; Jazz: Consultancy; Hanmi Pharm Co., Ltd.: Research Funding; Abbvie: Consultancy, Research Funding; NOHLA: Research Funding; Glycomimetics: Research Funding; Immunogen: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Agios: Consultancy; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Astellas: Consultancy; Genentech: Consultancy, Research Funding; Celgene: Consultancy. Cortes:Immunogen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy; Sun Pharma: Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding. Kadia:Celgene: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioline RX: Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. DiNardo:notable labs: Membership on an entity's Board of Directors or advisory committees; syros: Honoraria; medimmune: Honoraria; daiichi sankyo: Honoraria; abbvie: Consultancy, Honoraria; jazz: Honoraria; celgene: Consultancy, Honoraria; agios: Consultancy, Honoraria. Jabbour:Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Popat:Bayer: Research Funding; Incyte: Research Funding; Jazz: Consultancy. Oran:Astex pharmaceuticals: Research Funding; AROG pharmaceuticals: Research Funding. Konopleva:Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding. Garcia-Manero:AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding. Borthakur:Oncoceutics, Inc.: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; AbbVie: Research Funding; Eli Lilly and Co.: Research Funding; PTC Therapeutics: Consultancy; Oncoceutics: Research Funding; Novartis: Research Funding; Merck: Research Funding; Incyte: Research Funding; Cyclacel: Research Funding; GSK: Research Funding; Agensys: Research Funding; AstraZeneca: Research Funding; Bayer Healthcare AG: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Janssen: Research Funding; Strategia Therapeutics: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Xbiotech USA: Research Funding; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; NKarta: Consultancy; Arvinas: Research Funding; Polaris: Research Funding; Cantargia AB: Research Funding. Kantarjian:Novartis: Research Funding; Astex: Research Funding; Ariad: Research Funding; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Takeda: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding. Ravandi:Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Selvita: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Ricerche: Research Funding; Cyclacel LTD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal