Introduction: Acute myeloid leukemia (AML), a hematologic malignancy characterized by clonal expansion of abnormal myeloid progenitors, is a complex disease exhibiting a dynamic mutational landscape over time. Somatic mutations in the isocitrate dehydrogenase (IDH) 1 and 2 genes occur in ~20% of patients with AML, resulting in production of the oncometabolite D-2-hydroxyglutarate (2-HG). Ivosidenib (IVO), a mutant IDH1 (mIDH1) inhibitor, is approved in the US for mIDH1 relapsed or refractory (R/R) AML and newly diagnosed mIDH1 AML in patients ≥75 years old or with comorbidities precluding intensive induction chemotherapy. Durable remissions in mIDH1 R/R AML were achieved with IVO in a phase 1 study (NCT02074839), with a complete remission (CR) plus complete remission with partial hematologic recovery (CRh) rate of >30%, and a median duration of CR+CRh response of >8 months. In these patients, bulk next-generation sequencing (NGS) identified the most frequent baseline co-mutations as DNMT3A (34%), NPM1 (23%), and SRSF2 (20%), with mIDH2 detected in 2 of 101 (~2%) patients (DiNardo et al. N Engl J Med 2018). Though a recent case study (Harding et al. Cancer Discov 2018) described the appearance of mIDH2 in patients who relapsed to IVO (isoform switching), the frequency of this phenomenon is unknown. In addition, it is unclear whether mIDH2 and other co-occurring mutations exist within the same clone as mIDH1 at baseline and relapse, as dynamic clonal architecture cannot be precisely imputed by bulk NGS.
Aim: To define clonal architecture heterogeneity and pattern of mechanism of relapse at single-cell resolution in a subset of patients with mIDH2 detectable by bulk NGS following IVO treatment.
Methods: Single-cell targeted DNA sequencing (scDNA-seq) was performed on matched patient peripheral blood mononuclear cell (PBMC) samples at baseline and relapse, using a microfluidic platform (Tapestri®) with a 19-gene AML panel (Pellegrino et al. Genome Res 2018) capable of detecting rare subclones to a level of 0.1%. Data were processed and analyzed using Tapestri® Insights software and the timescape R package.
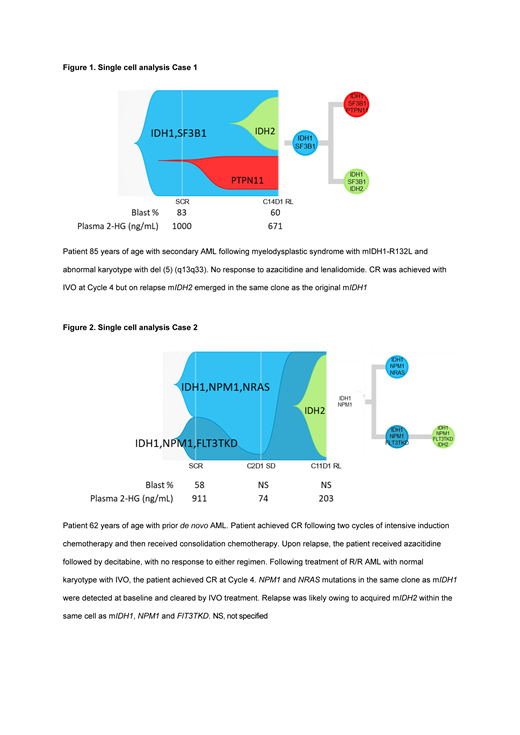
Results: Of 129 patients with available longitudinal genomic profiling data, 15 (12%) patients had detectable mIDH2 on treatment. Here we report findings from 9 of 15 patients with available scDNA-seq data. Seven of 9 patients had no detectable mIDH2 at baseline. Six of these 7 acquired mIDH2 at relapse within the same clone as the original mIDH1, whereas mIDH2 was identified in a separate clone to mIDH1 in 1 patient. In the 2 of 9 cases in which mIDH2 was detected at baseline, mIDH2 was present in a separate clone to mIDH1. AML-related gene mutations (e.g. PTPN11, NRAS, ASXL1) were also identified upon relapse following IVO treatment. Figure 1 demonstrates the emergence of mIDH2 in the same cell as mIDH1, concurrent with the expansion of a separate mIDH1 clone harboring a PTPN11 mutation at relapse. Figure 2 demonstrates two distinct mIDH1 clones at baseline, one harboring NPM1/NRAS co-mutations and the other harboring NPM1/FLT3-TKD co-mutations. Following IVO treatment, the IDH1/NPM1/NRAS clone was no longer detected. Reduction in the IDH1/NPM1/FLT3-TKD clone was observed at Cycle 2 Day 1, but it ultimately expanded at relapse with the acquisition of mIDH2. In both cases, plasma 2-HG was first inhibited by >95% but increased at relapse. Furthermore, phylogenetic tree reconstruction from clonotypes indicated patterns of both branching and linear clonal evolution.
Conclusions: In a subset of patients with mIDH2 detectable at relapse, mIDH2 was mostly not detectable at baseline but emerged within the same clone as mIDH1, highlighting 2-HG restoration as an important mechanism of resistance to IVO. Moreover, these data provide unique insights into the clonal dynamics in patients with mIDH1 R/R AML harboring mutations in the receptor tyrosine kinase (RTK) pathway, notably that the presence of RTK mutations at baseline does not universally preclude a clinical response. scDNA-seq proved to be a powerful tool in delineating molecular outcomes for patients with mIDH1 R/R AML. These findings, a part of emerging data highlighting the interplay between baseline mutation profiles and response and clonal evolution on treatment, support combinations or sequential treatment modifications at early relapse before overt clinical progression.
Wang:Agios: Employment, Equity Ownership. Choe:Agios: Employment, Equity Ownership. DiNardo:jazz: Honoraria; celgene: Consultancy, Honoraria; daiichi sankyo: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; abbvie: Consultancy, Honoraria; medimmune: Honoraria; agios: Consultancy, Honoraria; syros: Honoraria. Stein:Astellas Pharma US, Inc: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; PTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo, Inc.: Membership on an entity's Board of Directors or advisory committees; Bioline: Membership on an entity's Board of Directors or advisory committees. de Botton:Agios: Consultancy, Research Funding; Celgene: Consultancy, Speakers Bureau; Pierre Fabre: Consultancy; Servier: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Forma: Consultancy, Research Funding; Syros: Consultancy; Bayer: Consultancy; AbbVie: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Consultancy. Fathi:Agios, Astellas, Celgene, Daiichi Sankyo, Novartis, Takeda, Amphivena, Kite, Forty Seven,Trovagene, NewLink genetics, Jazz, Abbvie, and PTC Therapeutics: Consultancy; Amphivena, Kite, Jazz, NewLink Genetics,: Honoraria. Tallman:Biosight: Research Funding; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Patents & Royalties; ADC Therapeutics: Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellerant: Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Biosight: Research Funding; UpToDate: Patents & Royalties; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; UpToDate: Patents & Royalties; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellerant: Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellerant: Research Funding; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biosight: Research Funding; Cellerant: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellerant: Research Funding; Cellerant: Research Funding. Kantarjian:Ariad: Research Funding; Astex: Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Novartis: Research Funding; Agios: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; Jazz Pharma: Research Funding; Takeda: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding. Stone:AbbVie, Actinium, Agios, Argenx, Arog, Astellas, AstraZeneca, Biolinerx, Celgene, Cornerstone Biopharma, Fujifilm, Jazz Pharmaceuticals, Amgen, Ono, Orsenix, Otsuka, Merck, Novartis, Pfizer, Sumitomo, Trovagene: Consultancy; Argenx, Celgene, Takeda Oncology: Other: Data and Safety Monitoring Board/Committee: ; Novartis, Agios, Arog: Research Funding. Quek:Agios: Research Funding; Celgene: Research Funding, Speakers Bureau. Zhang:Agios: Employment, Equity Ownership; Agios: Employment, Equity Ownership. Liu:Agios: Employment, Equity Ownership. Attar:Aprea Therapeutics: Employment; Agios: Employment, Equity Ownership. Wu:Agios: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal