Background: The 2018 iwCLL guidelines recommend close observation of early stage CLL pts who do not meet indications for therapy ("watch and wait" strategy). Prior attempts at early therapeutic intervention in unselected early-stage CLL pts using alkylating agents such as chlorambucil and chemoimmunotherapy failed to show a significant benefit; and these therapies were associated with substantial toxicities. The introduction of Bruton tyrosine kinase (BTK) inhibitors has revolutionized the treatment landscape of CLL. The German CLL Study group recently presented results of the CLL12 study which demonstrated a significant improvement in event-free survival (EFS) among untreated early stage high risk CLL pts who received ibrutinib compared to placebo (median EFS not reached vs. 47.8 months, respectively; Langerbeins, EHA, 2019). Acalabrutinib is a more selective second-generation BTK inhibitor that has fewer adverse events in pts with CLL, and has shown equally impressive clinical activity for CLL pts. We are conducting an investigator initiated randomized phase 2 study to compare acalabrutinib with or without obinutuzumab (a glycoengineered anti-CD20 monoclonal antibody approved in the treatment of CLL) among high risk early stage CLL pts.
Study Design: All pts with newly diagnosed (<2 years from registration) previously untreated early stage CLL who do not meet the 2018 iwCLL guidelines for initiation of therapy are eligible for enrollment in this study. Pts undergo risk stratification according to the CLL-International prognostic index (CLL-IPI) which consists of the following 5 variables: age >65 years (1 point), Rai stage I-IV (1 point), del17p and/or TP53 mutation (4 points), unmutated immunoglobulin heavy chain (IGHV) genes (2 points), and serum β2-microglobulin >3.5 g/dL (2 points). Pts with high (4-6) and very high (7-10) risk CLL-IPI are randomly (1:1) assigned to acalabrutinib 100 mg orally twice daily (Arm A) or acalabrutinib with obinutuzumab at a standard approved schedule (Arm B). Treatment with acalabrutinib is continued for a minimum of 2 years unless the patient has unacceptable side effects or withdraws consent. Pts with low (0-1) and intermediate (2-3) risk CLL-IPI score are assigned to an observation arm with follow-up once every 6 months for 2 years, and then according to routine clinical practice (Arm C).
Endpoints: The primary endpoint of the intervention arms (Arms A and B) is the achievement of minimal residual disease (MRD)-negative complete remission in the bone marrow after 2 years of therapy. MRD is assessed using an 8-color flow cytometry with a detection limit of 0.01%. The primary endpoint of the observation arm (Arm C) is time to first therapy. Secondary endpoints for all arms are: progression-free survival, overall survival, and safety.
Statistical design: A randomized trial comparing the experimental arm (acalabrutinib plus obinutuzumab) against the control arm (acalabrutinib alone) will be conducted as described by Rubinstein. A sample size of 36 pts per arm provides 80% power to detect an improvement in MRD-negative complete response rate from 10% to 30%, using a one-sided test at a significance level of 0.10 (EAST 6.4). We anticipate accruing an additional 8 high/very high risk pts (4 in each arm) to account for ineligibility, cancellation, or major treatment violation. The total enrollment for Arms A and B will be 80 pts. We will enroll 40 pts to Arm C; for a total enrollment of 120 pts.
Key correlatives: Comprehensive profiling to assess the innate and adaptive immune system in paired peripheral blood and bone marrow will be conducted in all pts registered to Arms A and B at baseline, 12 and 24 months after enrollment. In addition, bone marrow hematopoietic function will be assessed in all pts at these time points. We will employ a CLL focused custom 61-gene panel to estimate tumor mutational burden as well as mutational profiles for each patient at baseline, 12, and 24 month time points, as well at the time of disease progression.
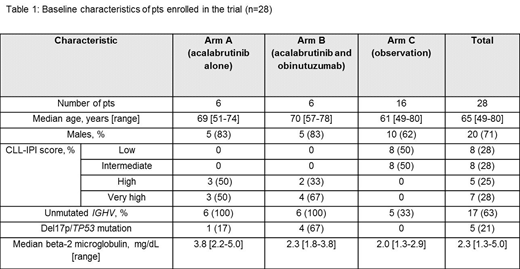
Preliminary results: The trial is registered at clinicaltrials.gov NCT03516617. The trial opened to accrual at all three Mayo Clinic sites (Rochester, MN; Jacksonville, FL; and Scottsdale, AZ) in September 2018. Twenty-eight pts have been registered; the baseline characteristics are shown in Table 1. The trial is expected to complete enrollment in September 2020; and the primary analysis will be available in September 2022.
Parikh:Janssen: Research Funding; AstraZeneca: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding; AbbVie: Honoraria, Research Funding; Acerta Pharma: Research Funding; Ascentage Pharma: Research Funding; Genentech: Honoraria. Ding:DTRM Biopharma: Research Funding; Merck: Research Funding. Ailawadhi:Pharmacyclics: Research Funding; Cellectar: Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy. Kenderian:Novartis: Patents & Royalties, Research Funding; Tolero: Research Funding; Humanigen: Other: Scientific advisory board , Patents & Royalties, Research Funding; Lentigen: Research Funding; Morphosys: Research Funding; Kite/Gilead: Research Funding. Chanan-Khan:Mayo Clinic: Employment; Ascentage: Research Funding; Millennium: Research Funding; Jansen: Research Funding; AbbVie: Research Funding; Xencor: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding. Kay:Infinity Pharmaceuticals: Other: DSMB; Celgene: Other: Data Safety Monitoring Board; Agios: Other: DSMB; MorphoSys: Other: Data Safety Monitoring Board.
Drug: Acalabrutinib Purpose: treatment of CLL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal