Background: Bruton tyrosine kinase (BTK) plays a critical role in B-cell receptor signaling, which mediates B-cell proliferation, migration, and adhesion. Inhibition of BTK has emerged as a strategy for targeting B-cell malignancies including CLL/SLL. Zanubrutinib (BGB-3111) is an investigational, next-generation BTK inhibitor that was designed to maximize BTK occupancy and minimize off-target inhibition of TEC- and EGFR-family kinases. Increased specificity may minimize toxicities reported with ibrutinib potentially due to off-target inhibition such as diarrhea, thrombocytopenia, bleeding, atrial fibrillation, rash, and fatigue (Coutre et al. Blood Advances 2019). In non-clinical studies, zanubrutinib has been shown to be highly potent, selective, bioavailable, and irreversible, with potentially advantageous pharmacokinetic (PK) and pharmacodynamic properties. Complete and sustained BTK occupancy has been observed with zanubrutinib treatment in both peripheral blood mononuclear cells and in lymph nodes (Tam et al. Blood 2019). Based on drug-drug interaction studies and population PK analyses (internal data), zanubrutinib may also be co-administered with strong or moderate CYP3A inhibitors at a reduced dose, proton pump inhibitors, vitamin K antagonists, as well as direct oral anticoagulants. Zanubrutinib does not prolong the QT interval. Pooled clinical data from 6 zanubrutinib monotherapy trials including 682 patients (pts) with either non-Hodgkin lymphoma, Waldenström macroglobulinemia, or CLL/SLL suggests that zanubrutinib has been generally well tolerated amongst pts with B-cell malignancies (Tam et al. EHA 2019). This data further showed that some toxicities often associated with BTK inhibitors were infrequent with zanubrutinib, including 1.9% atrial fibrillation/flutter (0.6% grade ≥3), 2.5% major hemorrhage (2.1% grade ≥3), 10.9% fatigue (0.7% grade ≥3), 18.0% rash (0.1% grade ≥3), 18.3% thrombocytopenia (6.6% grade ≥3), and 19.4% diarrhea (0.9% grade ≥3). Early clinical data in pts with treatment-naïve (TN; n=22) or relapsed/refractory (R/R; n=56) CLL/SLL showed that zanubrutinib was highly active: 96.2% overall response rate (ORR), including 4.5% and 1.8% with complete response in TN and R/R CLL/SLL, respectively (Tam et al. Blood 2019).
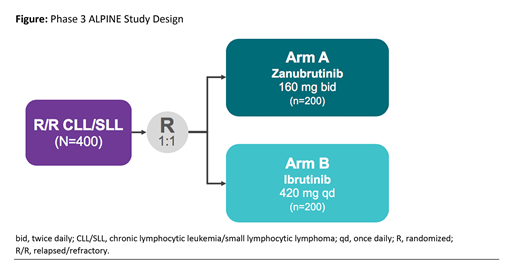
Study Design and Methods: This ongoing phase 3, randomized, global study (ALPINE; NCT03734016) is designed to compare the efficacy of zanubrutinib to ibrutinib based on ORR in pts with R/R CLL/SLL (Figure). This open-label study randomizes approximately 400 pts 1:1 to each arm, stratified by age (< 65 vs ≥ 65 years), refractory status (yes vs no), geographic region (China vs other), and del(17p)/TP53 mutation status (present vs absent). The study population includes adult pts who have had prior treatment for their CLL/SLL and were either refractory to or relapsed from prior CLL/SLL treatment. Major inclusion criteria include R/R CLL/SLL requiring treatment per International Workshop on CLL (iwCLL) criteria, disease measurable by computed tomography/magnetic resonance imaging, Eastern Cooperative Oncology Group performance status 0-2, and adequate hematologic function. Major exclusion criteria include prior treatment with a BTK inhibitor, current or past history of Richter transformation, clinically significant cardiovascular disease, or history of severe bleeding disorder. Zanubrutinib is dosed at 160 mg twice daily, and ibrutinib is dosed per label at 420 mg daily; treatment in both arms may continue until progression. The primary endpoint is ORR as determined by an independent review committee according to iwCLL guidelines, with modification for treatment-related lymphocytosis for pts with CLL and per 2014 Lugano Classification for pts with SLL. The study is powered to test the non-inferiority, and subsequently the superiority, of the ORR for zanubrutinib vs ibrutinib. Secondary endpoints include progression-free survival, ORR as determined by investigator, safety, duration of response, overall survival, and pt-reported outcomes. Exploratory endpoints include the correlation between clinical outcomes and prognostic and predictive biomarkers. Recruitment began in November 2018 and is ongoing in 14 countries.
Hillmen:Roche: Research Funding; Gilead: Research Funding; Apellis: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding. Brown:AbbVie: Consultancy; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Catapult Therapeutics: Consultancy; Dynamo Therapeutics: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy, Research Funding; Juno/Celgene: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy; Pharmacyclics: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy, Research Funding; Sun Pharmaceuticals: Research Funding; Janssen: Honoraria; Teva: Honoraria; Morphosys: Other: Data safety monitoring board; Invectys: Other: Data safety monitoring board; Octapharma: Consultancy. Byrd:Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; Novartis: Other: Travel Expenses, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Ohio State University: Patents & Royalties: OSU-2S; Ohio State University: Patents & Royalties: OSU-2S. Eichhorst:BeiGene: Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ArQule: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lamanna:Celgene: Consultancy; Oncternal: Research Funding; TG Therapeutics: Research Funding; Ming: Research Funding; Infinity/ Verastem: Research Funding. O'Brien:GlaxoSmithKline: Consultancy; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Acerta: Research Funding; Alexion: Consultancy; Regeneron: Research Funding; Eisai: Consultancy; Celgene: Consultancy; Aptose Biosciences, Inc: Consultancy; TG Therapeutics: Consultancy, Research Funding; Amgen: Consultancy; Kite: Research Funding; Janssen: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Verastem: Consultancy; Sunesis: Consultancy, Research Funding; Astellas: Consultancy; Vaniam Group LLC: Consultancy. Salmi:BeiGene: Employment. Hilger:BeiGene: Employment, Equity Ownership. Huang:BeiGene: Employment, Equity Ownership. Tam:AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie company: Honoraria; Novartis: Honoraria; BeiGene: Honoraria; Roche: Honoraria.
Zanubrutinib is an investigational agent and has not yet been approved in the US
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal