Background: Patients with acute myeloid leukemia (AML) presenting with hyperleukocytosis have frequent complications and early mortality. Pulmonary, central nervous system, and cardiovascular complications are common. Attempts to improve outcomes in the 10% of AML patients arriving with hyperleukocytosis have included leukapheresis. No prospective randomized study has supported the use of leukapheresis, and retrospective reports have revealed persistently poor outcomes as well as emerging concerns of acquired coagulopathy and worsening hypoxemia after leukapheresis. While many centers use a leukapheresis protocol processing two blood volumes, Johns Hopkins protocol routinely processes three-five blood volumes. This study aimed to assess coagulopathy, hypoxemia, and mortality with large volume leukapheresis.
Methods: 32 patients with newly diagnosed AML treated with large volume leukapheresis for WBC depletion are included in this report. Demographic, clinical, laboratory, and apheresis-related data were collected. Coagulopathy and hypoxemia-related metrics were evaluated within 6 hours before leukapheresis and within 6 hours of completion of leukapheresis. Descriptive and inferential statistics (chi square and Mann-Whitney U test) were used to compare pre and post-leukapheresis findings and assess clinical outcomes.
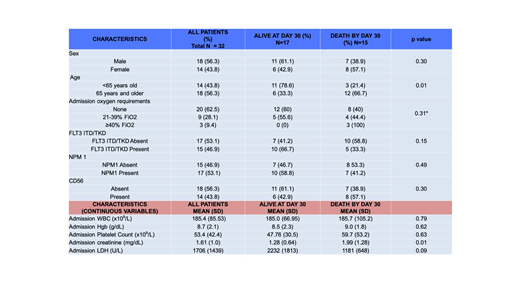
Results: Twenty-nine of 32 (93.8%) patients presented with symptomatic leukostasis (with pulmonary and/or CNS symptoms in 26/29). Median blood volume processed was 14.8 liters (range 4-23.4L). Mean platelet count decreased from 60x109/L to 37x109/L after leukapheresis (p<0.001). Comparing supplemental oxygen requirements before and after leukapheresis, 1 patient had decreased requirements, 6 increased, and 25 were unchanged. Median change in prothrombin time (PT) was an increase of 1 second (range: -2.4 to +7.7 seconds; p=0.13). Twenty-six of 26 evaluable patients had a decline in fibrinogen post-pheresis, with median reduction of 84 mg/dL (range: -12 to -483 mg/dL; p=0.04). Twelve of 18 (66.7%) patients 65 years and older died by day 30, compared with 3/14 <65 years-old (p=0.01). Fifteen of 32 (46.9%) arrived with acute renal failure on admission, 10 of whom died by day 30. All 8 patients requiring hemodialysis during their initial admission died prior to day 30. 12/32 (37.5%) required mechanical ventilation, 10 of whom died by day 30. Overall, 10/32 (31.3%) died by day 7, and 15/32 (46.9%) died by day 30. The most common primary cause of death was multiorgan failure including both renal failure and hypoxemia in 8 patients. Two patients died of confirmed intracranial hemorrhage, and 2 died with clinical suspicion for intracranial hemorrhage but were too unstable for imaging prior to death. Two deaths were attributed to ischemic stroke, and 1 patient died with isolated refractory hypoxemia.
Conclusions: Patients with AML presenting with hyperleukocytosis have a very high mortality, particularly when complicated by symptomatic leukostasis. Similar to Van de Louw's report, we observed worsening coagulopathy and a subgroup with increased oxygen requirements after leukapheresis. While our sample size is too small to draw broad conclusions, we were not able to identify a group clearly benefiting from leukapheresis. We did not find evidence that larger volume leukapheresis decreased complications or mortality. These results should lead to caution when considering leukapheresis for patients with newly diagnosed AML, particularly in those presenting with a severe coagulopathy.
Webster:Pfizer: Consultancy; Amgen: Consultancy; Genentech: Research Funding. Gojo:Amphivena: Research Funding; Amgen Inc: Consultancy, Honoraria, Research Funding; Juno: Research Funding; Merck: Research Funding; Jazz: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Pratz:Boston Biomedical: Consultancy; Millenium/Takeda: Research Funding; Agios: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Smith:Celgene: Consultancy; Jazz: Consultancy; Pfizer: Consultancy; Agios: Consultancy; Novartis: Consultancy. DeZern:Astex Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy. Levis:Amgen: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Astellas: Consultancy, Research Funding; FUJIFILM: Consultancy, Research Funding; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Daiichi Sankyo Inc: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal