Introduction: L-asparaginase has been an important component of acute lymphoblastic leukemia (ALL) therapy for over 30 years and is standard therapy during ALL remission induction and consolidation phases. L-asparaginase hydrolyzes the nonessential amino acid asparagine, depleting plasma levels and selectively killing cancer cells. Due to their bacterial origin, currently available L-asparaginases are immunogenic and can induce hypersensitivity reactions and high titers of neutralizing antibodies that may limit their therapeutic effect. The inability to receive asparaginase secondary to hypersensitivity has important prognostic implications for ALL patients (pts) and has been associated with significantly worse outcomes (Silverman LB, et al. Blood. 2001;97(5):1211-1218). Additionally, high-risk and slow early-responding standard-risk ALL pts who do not complete their prescribed asparaginase courses have a significantly inferior event-free survival (Gupta S, et al. J Clin Oncol. 2019;37:[suppl; abstract 10005]), demonstrating the negative effects of incomplete asparagine depletion. Therefore, to ensure that pts who develop dose-limiting hypersensitivity to E. coli-derived asparaginase products receive an adequate therapeutic course, similar alternative preparations are warranted. A recombinant crisantaspase such as RC-P, with no immunologic cross-reactivity to E. coli-derived asparaginase, may address this clinical need. JZP458-101 was a single-center, open-label, phase 1 study to evaluate the safety, tolerability, and pharmacokinetics (PK) of a single RC-P dose in healthy adults in order to facilitate the selection of a start dose and dosing regimen of RC-P for use in a pivotal phase 2/3 study in ALL pts.
Methods: Eligible subjects (sbj) were aged 18 to 55 years and in good general health as determined by the investigator. In Dose Cohort 1, sbj were randomized (1:1) to receive a single RC-P dose (25 mg/m2) by either a 2-hour intravenous (IV) infusion (N = 6) or an intramuscular (IM) injection (N = 6). After the safety, tolerability, and PK of RC-P in Dose Cohort 1 was evaluated to determine the need for another dosing cohort, Dose Cohort 2 randomized sbj (1:1) to receive a single RC-P dose of either 37.5 mg/m2 IV (N = 6) or 12.5 mg/m2 IM (N = 6). RC-P was administered in the inpatient clinical unit; sbj were discharged on Day 5 with safety follow-up calls on Days 6 and 30. The primary objective was to assess safety and tolerability of RC-P by IV and IM dosing for each cohort. Secondary objectives included characterization of RC-P PK by IV and IM administration based on serum asparaginase activity (SAA).
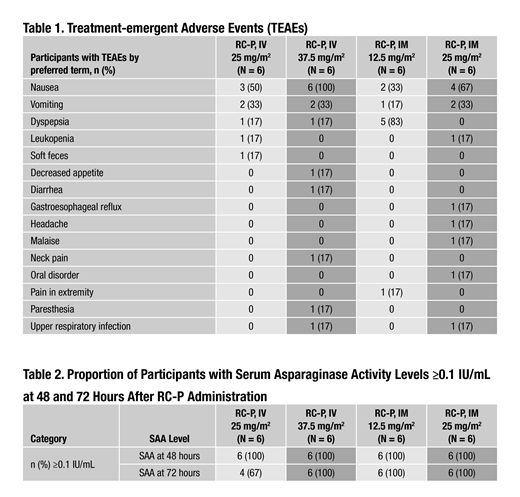
Results: Among the 24 RC-P sbj enrolled, demographic characteristics (mean ± SD) included: age (38.4 ± 8.30 years), weight (77.04 ± 10.00 kg), and body surface area (1.91 ± 0.15 m2). Additionally, 63% of sbj were male, 97% were of Hispanic/Latino ethnicity, 83% were white, and 17% were black/African American. Both safety and PK were evaluated in this study. For safety, 8/12 (67%) sbj had ≥1 adverse event (AE; IV = 4 sbj; IM = 4 sbj) in Dose Cohort 1. In Dose Cohort 2, 11/12 (92%) sbj had ≥1 AE (IV = 6 sbj; IM = 5 sbj). No serious AEs (SAEs) or grade ≥3 AEs were reported for any sbj in either dosing cohort. The most common treatment-emergent AE occurring in ≥2 sbj in each dosing cohort was nausea (Table 1). Dyspepsia was the most common AE in sbj who received RC-P 12.5 mg/m2 IM (Table 1). PK assessment showed that when administered IM, RC-P SAA levels achieved ≥0.1 IU/mL in 6/6 (100%) sbj at 48 and 72 hours post-dose in the 12.5 and 25 mg/m2 dose cohorts. Following IV administration, SAA levels achieved ≥0.1 IU/mL in 6/6 (100%) sbj at 48 hours and 4/6 (67%) sbj at 72 hours post-dose at the 25 mg/m2 dose level, while 6/6 (100%) sbj achieved ≥0.1 IU/mL at 48 and 72 hours post-dose at the 37.5 mg/m2 dose level (Table 2).
Conclusions: RC-P administration in healthy adults was well tolerated and there were no unanticipated AEs, no reported SAEs, and no grade ≥3 AEs. SAA levels ≥0.1 IU/mL, a surrogate marker for asparagine depletion, were achieved in all sbj receiving IM and IV RC-P at 48 hours. SAA levels ≥0.1 IU/mL were also achieved by all sbj at 72 hours after RC-P dosing, except for 2 sbj in the 25 mg/m2 IV group. Based on the totality of PK and safety data from this study, the recommended phase 2/3 starting dose is 25 mg/m2 for the IM route of administration and 37.5 mg/m2 for the IV route of administration on a Monday/Wednesday/Friday dosing schedule.
Hernandez-Illas:QPS Miami Research Associates: Employment. Lin:Jazz Pharmaceuticals: Employment, Equity Ownership. Rey:QPS Miami Research Associates: Employment. Jenkins:Jazz Pharmaceuticals: Employment. Chandula:Jazz Pharmaceuticals: Employment, Equity Ownership. Choi:Jazz Pharmaceuticals: Employment, Equity Ownership.
The abstract presents data from an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal