Myelodysplastic syndromes (MDS) are heterogeneous bone marrow failure neoplasms marked by cytopenias, reduced quality of life and predilection to transform into AML. While several treatments for AML have recently been approved, the available treatments for MDS are lacking, and adaptation of AML therapy to MDS is complicated. This is due, in part, to the heterogeneity of MDS. Despite this heterogeneity, most clonal cells in MDS have an imbalance of mitochondrial-controlled BCL2 family proteins resulting in dysregulated apoptosis. These anti- (or pro-) apoptotic proteins compete for ligand to block (or promote) apoptosis, providing an opportunity to selectively target anti-apoptotic proteins and advance therapy for MDS. Venetoclax (VEN), a newly FDA-approved therapy that specifically inhibits the anti-apoptotic protein BCL2, has yielded response rates of up to 50-70% in elderly AML including impressive responses in transformed MDS which previously failed DNMTi (DiNardo et al, 2019, Wei et al, 2019). Upregulation of the anti-apoptotic protein, induced myeloid cell leukemia-1 (MCL1), is a known resistance mechanism in AML resistant or refractory to BCL2 inhibition (Pan et al, 2014), and MCL1 increases when some MDS samples are treated with BCL2 inhibitors (Jilg et al, 2016). Therefore, strategic inhibition of BCL2 and/or MCL1 is a logical therapeutic approach in MDS. We have shown efficacy of MCL1 inhibitors in the laboratory against AML patient samples that are dependent on MCL1 protein or resistant to BCL2 inhibition, including AML cells that arose from MDS (Ramsey et al, 2018). Here, our goal was to determine the sensitivity of MDS cells to inhibition of specific anti-apoptotic proteins, elucidate the characteristic determinants of response, and investigate synergy with combined BCL2 and MCL1 inhibition.
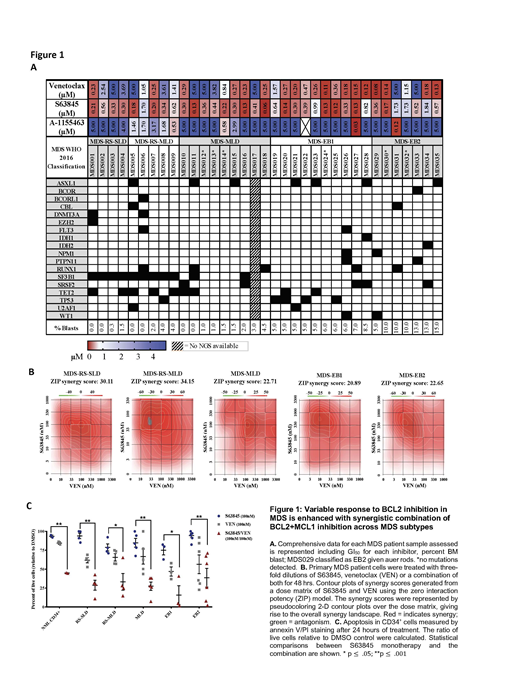
We cultured MDS patient samples and determined the in vitro sensitivity of 35 MDS patient samples to selective BCL2, BCL-XL and MCL1 inhibitors using CellTiter-Glo to determine the relative cell viability concentrations (GI50) for each selective inhibitor after 48 hours of exposure. While there was little sensitivity to BCL-XL inhibition across all samples, we detected a gradient of low to high response to the BCL2 inhibitor with low to higher blast count MDS subtypes; higher blast count MDS (EB1 and EB2) were more sensitive than low blast count subtypes (RS-SLD/MLD and MLD). Interestingly, nearly all MDS subtypes were sensitive to the selective MCL1 inhibitor, S63845. To determine if there were any correlations between sensitivity to specific inhibitors and mutational status, a targeted NGS panel of 37 commonly mutated genes in myeloid disease was conducted on all samples. As expected, we observed an increased number of SF3B1 mutations in the lower blast count MDS-RS patient samples. Likewise, though there were only two samples in this cohort containing mutations in PTPN11, one was completely resistant to BCL2 inhibition.(Stevens et al, ASH 2018) Otherwise, we did not observe any correlation between specific mutations and BH3 dependence. Since upregulation of MCL1 is seen in VEN treated MDS cells and is a known resistance mechanism for VEN treatment in AML, we treated these same patient samples with VEN+S63845 to determine any synergistic benefit of combining these drugs. While there were differential responses to VEN monotherapy between subtypes, all MDS subtypes exhibited response benefit to the addition S63845 to VEN. Drug synergy was confirmed with the Zero Interaction Potency model. These results were further corroborated by increased annexin-V staining and reduced colony formation in methylcellulose. Toxicity experiments in the MISTRG immunocompromised animal model indicate that the combination of selective inhibitors of MCL1 and BCL2 are tolerated, and treatment of MDS within these xenografts is underway and will be presented.
Overall, our data suggests that higher blast count MDS subtypes (EB1 and EB2) are more likely to respond to VEN monotherapy than low blast count subtypes, while all MDS subtypes may respond to MCL1 inhibition. Moreover, drug synergy can be obtained across all subtypes of MDS by combining BCL2 and MCL1 inhibitors. BCL2 inhibition is changing the standard of care in AML, thus, refining the design of clinical trials testing BCL2 and MCL1 inhibitors in MDS and the precision of patient selection for therapy is a great priority.
Savona:Boehringer Ingelheim: Patents & Royalties; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Research Funding; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Selvita: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal