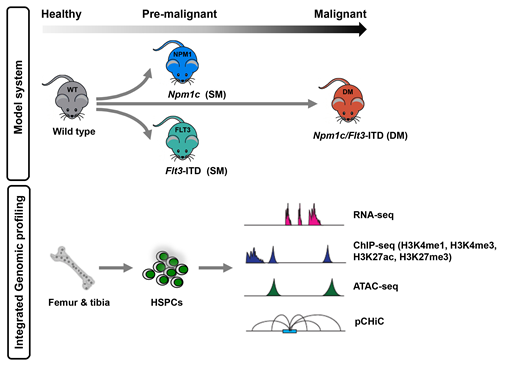
Aberrant transcriptional programs are cardinal features of Acute Myeloid Leukemia (AML). Recently, it has been shown that specific distal cis-regulatory elements called enhancers communicate with promoters through 3-D DNA looping to regulate tissue-specific gene expression. Recurrent mutations in epigenetic regulators that modify enhancers, transcription factors that bind enhancers and the structural proteins that promote DNA looping, such as the Cohesin complex and its major binding partner CTCF have been demonstrated in AML. However, how these mutations regulate chromatin and alter 3D-DNA topology and communication between enhancers and promoters to generate leukemia-specific transcriptional programs remains poorly understood. In addition, many AML cases lack mutations in epigenetic regulators, transcription factors or DNA structural proteins, yet still demonstrate aberrant transcription, suggesting indirect effects of other mutations on enhancer function and the epigenetic landscape. To address these questions, we have utilized an allelic series of mice carrying the most common mutations in AML, namely Flt3-ITD and Npm1c (co-mutated in ~15% of all AMLs). These model different "transition states" (normal: wild type (WT), Pre-Malignant: single mutant (SM) with either Flt3-ITD or Npm1c mutations and Malignant: double mutant (DM)) during AML induction. Moreover, our design allows analysis of the SM mice to deconvolute the contribution of individual mutations to altered chromatin regulation. We have analyzed hematopoietic stem and progenitor cells (HSPCs) from WT and mutant mice for gene expression (RNA-seq), chromatin activation states (ChIP-seq for H3K4me1, H3K4me3, H3K27ac and H3K27me3), chromatin accessibility (ATAC-seq), and promoter-anchored 3-D chromatin interaction (promoter capture HiC, pCHiC)(Figure 1) and have integrated these analyses to determine the transcriptional, epigenetic and DNA-topological evolution of AML.
Through pairwise comparisons between mutant and WT HSPCs, our data demonstrated that SM cells, with either Flt3-ITDor Npm1c mutations, alter gene expression only very modestly. However, when both mutations are present in DM cells, much larger gene programs that drive leukemia are both up- and downregulated. To examine the epigenetic regulation of these programs, we next built an enhancer compendium across all 4 allelic states using the H3K4me1 mark. Layering on H3K27ac activation, our data demonstrated that, in contrast to gene expression, significant alterations in enhancer specification and activation occur in advance of gene expression changes, to "prime" critical genes in Flt3-ITD, but not in Npm1c HSPCs. By contrast, Flt3-ITD and Npm1c mutations both altered global chromatin accessibility, with losses and gains evident at multiple critical genes. Similarly, our pCHiC data demonstrated significant alterations in DNA topology in mutant HSPCs that culminate in alterations in DNA "compartments" in DM HSPC. Moreover, they identified "hardwired" and "rewired" interactions between promoters and enhancers important for expression of critical leukemia programs. Analyses of all of these separate layers demonstrated a uniform pattern; progressive alterations in the transition from SM to DM HSPCs. Integrating these layers of analysis clearly demonstrated synergy between the mutations and a correlation between gene expression changes and chromatin dynamics in mutant cells. Furthermore, performing de novo motif analysis suggested a signal-specific transcription factor (TF) network downstream of Flt3-ITD that was amplified in the DM HSPC and that was corroborated by GSEA analysis. Our data had identified long-range regulatory control regions at the Spi1/PU.1 and Hoxa cluster loci amongst many others, and motif analysis had suggested Hox and Pu.1 to be important TFs in our malignant networks. Using these as examplars, we then perturbed the genes and regulatory elements at these loci by shRNA knockdown and CRISPR-mediated excision and could abrogate leukemic growth, validating the importance of our proposed networks. Taken together, these integrated analyses demonstrate a highly dynamic and coordinated process, where the effects of individual mutations synergize to remodel the chromatin landscape and 3D-DNA topology to generate networks that initiate and maintain AML transcriptional programs.
Vassiliou:Kymab Ltd: Consultancy, Other: Minor Stockholder; Oxstem Ltd: Consultancy; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal