Introduction: Outcomes of adverse risk acute myeloid leukemia (AML) remain dismal. Despite some morphologic remission following therapy, the majority of patients relapse and succumb to their disease. Induction chemotherapy leads to a significant reduction in tumor burden, however, resistant leukemia cells persist as minimal residual disease (MRD), the reservoir for relapse. This is likely due to the capacity of these persistent cells to hijack properties from normal hematopoietic stem cells such as self-renewal, quiescence, and recapitulation of the malignant progeny. Thus leukemia cells are functionally heterogeneous, with the majority of cells at diagnosis susceptible to chemotherapy, and a minority of resistant cells that persist despite treatment. Deeper understanding of all leukemia sub-populations is necessary in order to understand mechanisms of resistance. We hypothesized that sub-populations such as leukemia-stem cells (LSCs), and post-therapy residual cells possess identifiable, targetable characteristics that drive resistance. We performed RNA-sequencing and compared differences in gene expression between these sub-populations.
Methods: We collected 47 bone marrow samples from 27 patients who met criteria for adverse risk AML by ELN 2017 risk stratification. We performed RNA-sequencing on paired pre- and post-treatment sorted samples. Mononuclear cells were flow-sorted for bulk (CD45dim) and LSCs (Lin-CD34+CD38-CD123+) from diagnostic samples. Post-treatment samples were sorted for bulk mononuclear cells and MRD, determined based on patient-specific aberrant phenotype using multi-color flow cytometry analysis (Xu J et al., Clinics in laboratory medicine 2017). Sixteen patients (59%) had mutations in TP53, 9 (33%) had mutations in FLT3, and 3 (11%) had no mutations in these genes but had other adverse risk features. RNA was isolated using low-input methodology, and RNA-sequencing was performed using Illumina HiSeq 2000. Samples with low-expression of housekeeping genes were excluded from the analysis. Differential expression was analyzed using DESeq2 and Gene Set Enrichment Analysis (GSEA) was performed using the HALLMARK gene set.
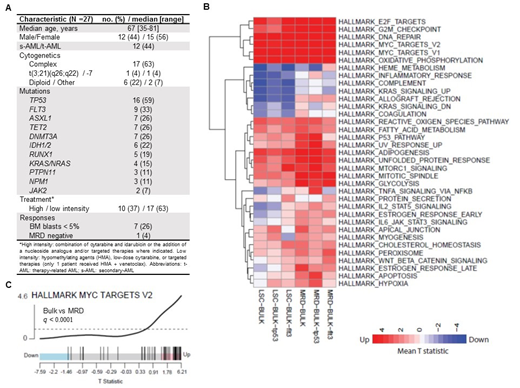
Results: The median age of patients included in this cohort was 67 years (range: 35-81). Baseline characteristics, including adverse risk features, commonly mutated genes, treatments and responses are described in Figure 1A. Differentially expressed genes were compared between sub-populations. Figure 1B includes pathways with statistically significant changes (changes with q<0.1 in at least one comparison are plotted in the heat map). Up-regulation of Myc-related genes was found when comparing bulk to LSCs or to MRD regardless of the genetic context (TP53 or FLT3 mutated) (Figure 1C). Similarly, there was up-regulation of genes related to the transcription factor E2F, to cell cycle checkpoints and DNA repair pathways. In addition, up-regulation of oxidative phosphorylation was found in both LSC and post-treatment MRD. This is consistent with previous data showing dependence of LSCs and cytarabine-resistant AML cells on mitochondrial function (Lagadinou et al., Cell Stem Cell 2013; Farge et al., Cancer Discovery 2017). On the other hand, we found down-regulation of immune-related genes in LSCs compared to bulk (allograft, inflammatory response, and complement-related gene sets). This is consistent with the potential of AML LSCs to evade the immune system regardless of the genetic context in this cohort. Interestingly, post-treatment MRD, in the TP53 mutated sub-group only, had up-regulation of TNFa-signaling pathway genes. This could be a specific mechanism by which AML cells with TP53 mutations modulate and evade immune control following treatment.
Conclusions: In conclusion, we show that aberrant transcriptional changes may account for resistance to therapy in adverse risk AML. The transcriptome of pre-treatment LSCs and post-treatment MRD is characterized by up-regulation of Myc-related genes, cell cycle checkpoints, DNA repair pathways, and oxidative phosphorylation. We also identified down-regulation of immune-related genes in LSCs. These findings have potential impact on future therapeutic strategies aimed at overcoming resistance in adverse risk AML.
Konopleva:Agios: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Astra Zeneca: Research Funding; Cellectis: Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ablynx: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Kisoji: Consultancy, Honoraria; Ascentage: Research Funding. Andreeff:Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Oncoceutics: Equity Ownership; BiolineRx: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Equity Ownership; Breast Cancer Research Foundation: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership; Aptose: Equity Ownership; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees; CPRIT: Research Funding; NIH/NCI: Research Funding; Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Amgen: Consultancy; AstaZeneca: Consultancy; 6 Dimensions Capital: Consultancy; Reata: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal