Background: Patients (pts) with MDS or AML who relapse after allogeneic transplantation (allo-HCT) have a very poor prognosis. Hypomethylating agents (HMA) and checkpoint blockade with the anti-CTLA4 blocking antibody ipilimumab (IPI) have each induced responses with acceptable toxicity in AML pts who relapse after allo-HCT. We hypothesized that adding decitabine (DAC) would improve response compared with IPI alone by activating and promoting T cell-mediated anti-leukemic immune reactivity. We are conducting a multicenter phase I study (CTEP 10026) of DAC plus IPI in pts with R/R MDS/AML in both post allo-HCT and transplant-naïve settings to assess safety and estimate efficacy.
Methods: The primary objective is to determine the maximum tolerated dose (MTD) or RP2D of combination DAC + IPI in pts with R/R MDS/AML who are post allo-HCT (Arm A) or transplant-naïve (Arm B). Cohorts of 3-6 are sequentially enrolled in 3 dose levels (DL) of IPI using a 3+3 design with expansion in each arm; DLs 0-2 are 3, 5 and 10 mg/kg, respectively. Eligibility for both arms: relapsed AML (extramedullary or ≥ 5% blasts) or R/R MDS (≥ 5% blasts) or unfit elderly AML; Arm A only: ≥ 2 wks off systemic immunosuppressive (IS) therapy, T cell chimerism ≥ 20%, and no prior acute GVHD ≥ gr III. DLT is defined as ≥ gr 3 non-heme, ≥ gr 3 acute GVHD or ≥ gr 3 steroid-refractory immune-related adverse events (AEs) occurring within 8 weeks from first IPI dose. Epigenetic priming with DAC lead-in cycle 0 was followed by combination cycles of DAC + IPI. DAC is given at 20 mg/m2 days 1-5 q 28 days. IPI is given on day 1 of cycles 1-4 and every other cycle in cycles 5-12. Pts who discontinued study either in cycle 0 or DLT period without IPI-toxicity were replaced. Arm A opened after safety was confirmed at DL0 in Arm B.
Results: As of June 9, 2019, 26 pts (15M, 11 F) have enrolled in this on-going trial.
Of the 12 pts (11 AML and 1 MDS) enrolled in Arm A (post allo-HCT), median age was 66.5 (range 29-74) and 9 had previously received HMA. 7 of 8 pts in DL0 (1 progressed in cycle 0) and 3 of 4 pts in DL1 (1 died from pneumonia in cycle 0) received DAC + IPI. DL0 was expanded to 6 to confirm safety without DLT. Median treatment duration after first IPI dose was 5 cycles (range 1-7); 4 pts continue on trial. Common AEs were gr 1-2 dyspnea (n=4), gr 1-3 fatigue (n=4), and gr 1-2 fever (n=4). Gr 3 AEs were febrile neutropenia (n=2), pneumonia (n=1), and candidemia (n=1). Gr 1 immune-related dermatitis (n=1) reversed with steroids. Acute GVHD was not observed. Moderate-severe chronic GVHD was noted in 2 pts mainly involving skin, which was responsive to photopheresis and oral IS. Though 1 CR and 1 marrow CR have been observed at DL0, dose-escalation up to DL2 is on-going to determine MTD.
Of the 14 pts (11 AML and 3 MDS) enrolled in Arm B (transplant naïve), median age was 75.5 (range 34-82) and 9 had previously received HMA. 4 of 6 pts in DL0 (1 progressed and 1 withdrew in cycle 0), 3 of 5 pts in DL1 (2 withdrew in cycle 0) and 3 of 3 pts in DL2 received DAC + IPI. Median treatment duration after first IPI dose was 4 cycles (range 1-8); 3 pts remain on study. Common AEs were gr 1-3 fatigue (n=9), gr 1-2 anorexia (n=5), and gr 3 febrile neutropenia (n=8). Immune-related gr 2 colitis (n=1) and gr 2/3 (n=4) dermatitis were all steroid-responsive. Of the 10 pts who received at least one IPI dose, 5 (50%) achieved an objective response including 3 CR, 1 CRi and 1 PR. All responses were observed in AML pts, including 1 with only skin involved. Expansion to confirm MTD is underway.
No treatment-related deaths or DLTs were observed in either Arm. Reasons for discontinuation after IPI: progression (n=9), proceeding to allo-HCT or DLI (n=2), withdrawal (n=1), stroke due to underlying atrial fibrillation (n=1) and disseminated nocardiosis (n=1).
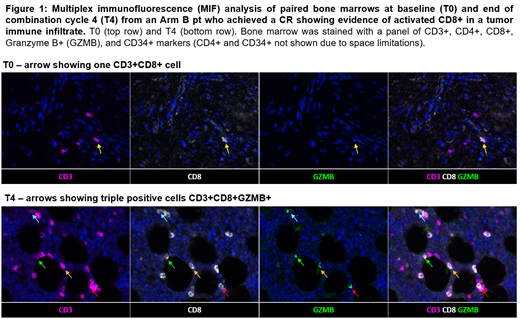
In both Arms, multiplex immunofluorescence (MIF) staining of BM biopsies revealed a higher density of CD3+CD4+ cells after 4 cycles of DAC + IPI in 4 responders (R) compared to 4 non-responders (NR) (p=0.0433). Longitudinal MIF IHC in an Arm B responder identified the increasing presence of a tumor immune infiltrate composed of CD3+CD8+GZMB+ T cells prior to achieving CR (Fig 1).
Conclusions: Combination DAC + IPI is tolerable and has encouraging clinical activity in post allo-HCT and transplant naïve pts with R/R MDS/AML. Ongoing studies focus on comparing the immunologic and genetic characteristics of the tumor immune infiltrate in each cohort to understand the contribution of alloimmunity to treatment response.
Garcia:Abbvie: Research Funding; Genentech: Research Funding. Keng:agios: Membership on an entity's Board of Directors or advisory committees. Brunner:Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Forty Seven Inc: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Research Funding. Khaled:Omeros: Consultancy; Alexion: Consultancy, Speakers Bureau; Daiichi Sankyo: Other: Travel support. Steensma:H3 Biosciences: Other: Research funding to institution, not investigator.; Arrowhead: Equity Ownership; Onconova: Consultancy; Stemline: Consultancy; Aprea: Research Funding; Pfizer: Consultancy; Summer Road: Consultancy; Astex: Consultancy. Winer:Jazz Pharmaceuticals, Pfizer: Consultancy. Cutler:Omeros: Consultancy; Kadmon: Consultancy; BiolineRx: Other: DSMB; Cellect: Other: DSMB; Kalytera: Other: DSMB; ElsaLys: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy; Fate Therapeutics: Consultancy; Incyte: Consultancy; Jazz: Consultancy; BMS: Consultancy. Ho:Omeros Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Consultancy. Neuberg:Madrigal Pharmaceuticals: Equity Ownership; Pharmacyclics: Research Funding; Celgene: Research Funding. Lindsley:Takeda Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Research Funding; Medlmmune: Research Funding. Galinsky:ABIM: Other: Member of specialty oncology board; Merus Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; AbbVie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Ritz:TScan Therapeutics: Consultancy; LifeVault Bio: Consultancy; Kite Pharma: Research Funding; Talaris Therapeutics: Consultancy; Draper Labs: Consultancy; Avrobio: Consultancy; Celgene: Consultancy; Merck: Research Funding; Equillium: Research Funding; Aleta Biotherapeutics: Consultancy. Davids:AbbVie, Acerta Pharma, Adaptive, Biotechnologies, Astra-Zeneca, Genentech, Gilead Sciences, Janssen, Pharmacyclics, TG therapeutics: Membership on an entity's Board of Directors or advisory committees; AbbVie, Astra-Zeneca, Genentech, Janssen, MEI, Pharmacyclics, Syros Pharmaceuticals, Verastem: Consultancy; Acerta Pharma, Ascentage Pharma, Genentech, MEI pharma, Pharmacyclics, Surface Oncology, TG Therapeutics, Verastem: Research Funding; Research to Practice: Honoraria. Wu:Pharmacyclics: Research Funding; Neon Therapeutics: Other: Member, Advisory Board. Stone:AbbVie, Actinium, Agios, Argenx, Arog, Astellas, AstraZeneca, Biolinerx, Celgene, Cornerstone Biopharma, Fujifilm, Jazz Pharmaceuticals, Amgen, Ono, Orsenix, Otsuka, Merck, Novartis, Pfizer, Sumitomo, Trovagene: Consultancy; Argenx, Celgene, Takeda Oncology: Other: Data and Safety Monitoring Board/Committee: ; Novartis, Agios, Arog: Research Funding. DeAngelo:Blueprint: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Research Funding; Glycomimetics: Research Funding; Amgen, Autolus, Celgene, Forty-seven, Incyte, Jazzs, Pfizer, Shire, Takeda: Consultancy. Soiffer:Jazz: Consultancy; Gilead, Mana therapeutic, Cugene, Jazz: Consultancy; Juno, kiadis: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Kiadis: Other: supervisory board; Mana therapeutic: Consultancy; Cugene: Consultancy.
Combination of ipilimumab and decitabine for MDS/AML treatment for patients who are post-transplant or transplant naive
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal