Introduction: Selinexor is a novel, oral selective inhibitor of nuclear export (SINE) which forces nuclear retention and activation of tumor suppressor proteins. Selinexor plus low dose dexamethasone (Sel-dex) was recently approved in the United States based on data from the STORM study wherein, Sel-dex induced an overall response rate (ORR) of 26.2% in patients with penta-exposed, triple-class refractory multiple myeloma. The presence of high-risk cytogenetic abnormalities is known to have a poor prognosis in multiple myeloma, with transient responses. We performed post-hoc analyses to determine the outcomes in patients with relapsed/refractory myeloma treated with Sel-dex based on the baseline cytogenetic abnormalities.
Methods: STORM was a phase 2b, open-label study which enrolled patients with relapsed and refractory myeloma. Selinexor 80 mg in combination with dexamethasone 20 mg was administered orally, twice weekly. The primary endpoint was ORR. For this analysis, we pooled 78 patients (48 quad-refractory and 30 penta-refractory) from STORM, part 1 and 122 patients (penta-exposed and triple class refractory) from STORM, part 2 to compare outcomes between high-risk and standard risk cytogenetics groups. High-risk cytogenetics was defined as having at least 1 of the following abnormalities by fluorescence in-situ hybridization (FISH) at baseline: del(17p), t(4;14), t(14;16), and gain(1q) in >5% of screened plasma cells. The FISH analyses were performed at a central laboratory and used to assess cytogenetic risk status.
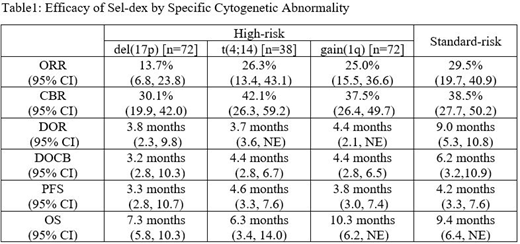
Results: Of the 200 patients, 122 (61%) had high-risk disease (del(17p): 36%, t(4;14): 18%, t(14;16): 5%, and gain(1q): 36%). The ORR in high-risk patients was 20.5% (very good partial response [VGPR)]: 5.7% and partial response [PR]: 14.8%) and the ORR was 29.5 % (complete response [CR]: 2.6%, VGPR: 3.8%, and PR: 23.1%) in standard-risk patients. The clinical benefit rate (CBR) was 35.2% in high-risk patients compared with 38.5% in standard-risk patients. Median duration of clinical benefit (DOCB) was 4.4 and 6.2 months in the high-risk and standard-risk patients respectively. Median progression-free survival (PFS) was 3.8 and 4.2 months and overall survival (OS) was 8.6 and 9.4 months in the high-risk and standard-risk patients, respectively. Efficacy by specific cytogenetic abnormality is presented in Table 1 below (Due to the small sample size (n=11), data for the t(14;16) subgroup are not presented separately).
Conclusions: Sel-dex demonstrated a similar CBR in patients with high risk and standard risk disease and preserved clinical benefit in heavily pre-treated patients who had rapidly proliferative disease and high-risk cytogenetics at baseline. The benefit was maintained across cytogenetic risk subgroups with a higher ORR in the t(4;14) and gain(1q) subgroups. The DOR and OS was similar across all the subgroups. These analyses support the use of Sel-dex in patients with high-risk cytogenetics and warrant further evaluation of selinexor in combination with other anti-myeloma therapies in high-risk disease.
Nooka:GSK: Honoraria, Other: advisory board participation; Amgen: Honoraria, Other: advisory board participation; BMS: Honoraria, Other: advisory board participation; Janssen: Honoraria, Other: advisory board participation; Spectrum pharmaceuticals: Honoraria, Other: advisory board participation; Adaptive technologies: Honoraria, Other: advisory board participation; Celgene: Honoraria, Other: advisory board participation; Takeda: Honoraria, Other: advisory board participation. Yee:Adaptive: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Huff:Karyopharm, Sanofi, MiDiagnostics: Consultancy; Member of Safety Monitoring Board for Johnson and Johnson: Membership on an entity's Board of Directors or advisory committees. Vogl:Karyopharm Therapeutics: Consultancy; Takeda: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Active Biotech: Consultancy. Chari:Novartis Pharmaceuticals: Research Funding; GlaxoSmithKline: Research Funding; Array Biopharma: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Oncoceutics: Research Funding. Moreau:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Dingli:Karyopharm: Research Funding; Rigel: Consultancy; Millenium: Consultancy; alexion: Consultancy; Janssen: Consultancy. Lonial:Genentech: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy, Research Funding; Amgen: Consultancy; BMS: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy; Celgene Corporation: Consultancy, Research Funding. Dimopoulos:Sanofi Oncology: Research Funding. Vij:Takeda: Honoraria, Research Funding; Sanofi: Honoraria; Karyopharm: Honoraria; Janssen: Honoraria; Genentech: Honoraria; Celgene: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Tuchman:Karyopharm: Honoraria; Celgene: Honoraria, Research Funding, Speakers Bureau; Amgen: Research Funding; Merck: Research Funding; Prothena: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Alnylam: Honoraria, Research Funding. Biran:Bristol Meyers Squibb: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Siegel:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Richardson:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Liu:Karyopharm Therapeutics: Employment, Equity Ownership. Joshi:Karyopharm Therapeutics: Employment, Equity Ownership. Shah:Karyopharm Therapeutics: Employment, Equity Ownership. Shacham:Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Kauffman:Karyopharm Therapeutics Inc: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Jagannath:Medicom: Speakers Bureau; Multiple Myeloma Research Foundation: Speakers Bureau; BMS: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal