Background: Immune aplastic anemia (AA) is caused by cytotoxic T cells (CTLs) that destroy hematopoietic stem and progenitor cells. Regulatory T cells (Tregs) are reduced in AA and increase in response to immunosuppressive therapy (IST; Solomou E et al, Blood 2007). Recent studies suggested an immune regulatory role of regulatory B cells (Bregs). Human CD19+CD24hiCD38hi Bregs suppress Th1 response of CD4+ T cells as well as IFN-γ production by CD8+ CTLs (Mauri C, Menon M, J Clin Invest 2017). The quantity and/or function of Bregs are impaired in autoimmune diseases, malignancies, chronic graft-versus-host disease, and during rejection of transplanted organs.
Methods: We investigated B cell phenotypes including CD24hiCD38hi Bregs in previously untreated severe AA (SAA) and very severe AA (VSAA) patients, and healthy individuals aged 18 years and older, and tested their correlation with severity and response to IST. Absolute numbers of lymphocyte subsets, including CD19+ B cells, CD8+ T cells, CD4+ T cells, and NK cell (TBNK), were quantified in fresh blood. Percentages of B cell subsets among total CD19+ B cells, including CD24hiCD38hi Bregs, CD24loCD38lo mature naïve B cells, CD24hiCD38lo memory B cells and CD24loCD38hi plasma cells/plasmablasts, were analyzed using cryopreserved peripheral blood mononuclear cells (PBMCs). Blood samples were obtained from patients close to time of diagnosis and before institution of definitive therapy. All patients were treated with horse anti-thymocyte globulin, cyclosporine, and eltrombopag between 2012 and 2018 at the Hematology Branch, NHLBI (clinicaltrials.gov NCT01623167).
Results: TBNK analysis revealed no significant difference in total B cell counts in 104 AA patients compared to 40 healthy individuals (median, 137/μl [IQR, 73-212] vs 163/μl [106-242], P=.11); NK cells were significantly decreased in patients with AA, as previously reported (Gascon P et al, Blood 1986). Total B cell count did not correlate with severity of AA (P=.89) nor with overall response at six months (P=.93). CD8+ T cells and NK cells were lower in VSAA patients compared to SAA patients. None of the TBNK subsets was predictive of overall response in six months after IST.
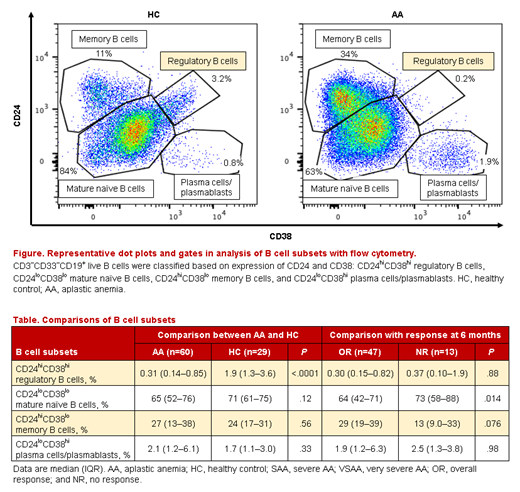
When we assessed the phenotype of B cells among 60 AA patients whose cryopreserved PBMCs were available, CD24hiCD38hi Bregs were markedly decreased as compared to 29 healthy individuals (0.31% [0.14-0.85%] vs 1.9% [1.3-3.6%], P=3×10-7; Figure, Table), while there was no significant difference in other B cell phenotypes. Among these 60 patients, the percentage of CD24hiCD38hi Bregs was especially decreased in VSAA patients compared to SAA (0.18% [0.11-0.34%] vs 0.50% [0.17-1.4%], P=.017). In contrast, CD24loCD38lo mature naïve B cells were higher in VSAA than in SAA (69% [58-86%] vs 60% [42-70%], P=.024). CD24hiCD38hi Breg frequency was positively associated with neutrophil and reticulocyte counts (correlation coefficients [r], 0.34 and 0.26, respectively), while the frequency of CD24loCD38lo mature naïve B cells was negatively correlated (r, -0.34 and -0.40). CD24loCD38lo mature naïve B cells before IST were significantly lower in 47 patients who achieved overall responses at six months compared to 13 non-responders (64% [42-71%), vs 73% [58-88%], P=.014), but CD24hiCD38hi Breg frequency was not correlated with IST responses. At six months after IST, CD24hiCD38hi Bregs in AA patients had recovered to levels present in healthy individuals (2.3% [0.98-4.8%]), in both 34 responders and five non-responders; non-responders showed non-significant increased CD24loCD38lo mature naïve B cells at six months (P=.068).
Discussion: A deficit of circulating CD24hiCD38hi Bregs in immune AA with recovery after IST, as occurs with Tregs, suggests Bregs may contribute to the immune pathophysiology in AA. We unexpectedly observed a higher percentage of CD24loCD38lo mature naïve B cells to be associated with more severe disease and a lower probability of responses to IST. B cell phenotype analysis may be beneficial for monitoring of AA and predicting outcomes of therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal