TO THE EDITOR:
Novel immunotherapies have recently led to a broadened spectrum of therapeutic options in pediatric B-cell precursor acute lymphoblastic leukemia, even in the setting of relapsed/refractory disease. However, antibody therapy for T-cell acute lymphoblastic leukemia (T-ALL) is currently nonexistent, and no antibody has been approved for clinical use in that entity. Bride et al have recently reported in Blood that the CD38-targeting antibody daratumumab (DARA), approved for the treatment of multiple myeloma, has preclinical efficacy in T-ALL patient-derived xenografts (PDXs) in nonobese diabetic/severe combined immunodeficiency/Il2rgtm1wjl/SzJ (NSG) mice.1 Off-label use has been reported in 1 adult T-ALL patient with post-stem cell transplantation relapse in whom the relapse could be salvaged with DARA.2 Here, we asked whether the addition of DARA could enhance the efficacy of chemotherapy mimicking ALL induction in a preclinical PDX model of T-ALL. Furthermore, we aimed to assess depth of remission achievable with DARA and whether CD38 expression levels were correlated with therapy response.
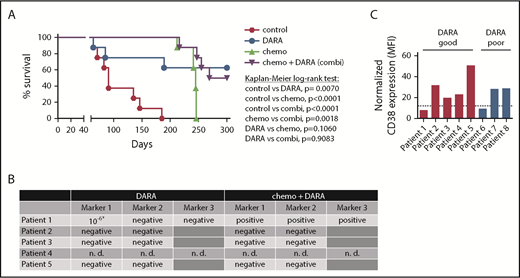
We first analyzed the efficacy of DARA as a single agent and DARA in combination with chemotherapy in 8 T-ALL patient samples from the ALL-BFM 2000 study in a randomized preclinical phase 2-like xenograft trial (Table 1). Randomized phase 2-like xenograft trials as previously published for small-molecule inhibitors by others3 and therapeutic antibodies by our group4 are a good way to depict clinical reality, as heterogeneity in patient populations is reflected. The trial was designed to detect a survival difference of 85% between DARA and untreated control mice at 150 days, in which the anticipated survival is 10% in the control group by that time. Statistical testing (χ2 test) yielded a group size of n = 8. Mice were left untreated (control); treated with DARA (8 mg/kg body weight intraperitoneally on days 1, 3, 6, 10, and 13 after injection) as previously published4,5 ; treated with chemotherapy comprising dexamethasone, vincristine, and pegylated Escherichia coli asparaginase (chemo); or treated with the combination of both (chemo+DARA). Median survival in the control group was 91 days and could be significantly prolonged by the application of up to five cycles of chemotherapy (P < .0001, Kaplan-Meier log-rank test; Figure 1A). However, all chemotherapy-treated animals had to be sacrificed due to symptomatic leukemia between days 241 and 246 after therapy initiation despite the heterogeneity of the samples. In the DARA group, 3 out of 8 mice (38%) succumbed to the disease between days 64 and 189. Monitoring of human leukemia in the blood showed an initial response in 2 of these 3 mice (50% vs 11% patient 6 and 24% vs 2% patient 8 at time point 1), which could however not be maintained (supplemental Figure 1, available on the Blood Web site). Survival prolongation in the DARA group was statistically significant as compared with control (P = .007; Figure 1A). Importantly, 4 out of 8 animals (50%) in the chemo+DARA group succumbed to the disease, but at later time points. All other animals in the combination group showed long-term survival and absence of clinical symptoms (P < .0001 as compared with control; Figure 1A). Notably, all of the PDX mice showing long-term survival with the combination showed the same with DARA alone. The experiment was terminated on day 300 due to legal requirements. All mice sacrificed before that time point showed extensive leukemic engraftment (supplemental Figure 1).
Characteristics of the T-ALL patients from the ALL-BFM 2000 study used for the randomized phase 2-like preclinical study
| Parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . |
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, y | 10 | 8 | 16 | 7 | 8 | 13 | 2 | 16 |
| Sex | M | M | M | F | M | M | M | M |
| WBC count (initial), ×109/L | 687 | 303 | 188 | 127 | 166 | 202 | 672 | 76 |
| Blast (PB) initial, % | 80 | 94 | 89 | 94 | 74 | 80.5 | 96.5 | 58 |
| Blast (BM) initial, % | No data | 98 | 88 | 98 | 96 | 84.5 | No data | 92 |
| Immunophenotype | Cortical T | Cortical T | Cortical T | Cortical T | Cortical T | Cortical T | Pre-T | Cortical T |
| Karyotype | 46,XY, t(X;9)(q22;p12), del(6)(q24)[15] | No MP | No MP | 48,XX,+4,+8[15] | 47,XY,-5, del(6)(q23),+9, add(17)(q21), +mar[11] | 46,XY, t(1;14)(p32∼33;q11) [15] | 46,XY, t(1;6)(p34;q22), del(9)(p21)[12]/46,XY[3] | 46,XY, del(4)(q31), add(7)(q34), add(15)(q13), inc[3]/46,XY[12] |
| Transcription factors (quantitative PCR*) | TAL1, LMO2, LYL | LMO1 | LMO2, LYL, MYB | HOXA9, HOXA10, LMO2, LYL, MYB | TAL1, LMO2, LYL | TAL1, LMO2 | LMO2, LYL | TAL1 |
| Mutations† | None detected | Notch HD-N1 | Notch PEST 1 | PTEN Ex 7 | WT1 Ex7 | Notch PEST 1 | Stat5B Ex16 | Fbxw7 Ex 9+10 |
| WT1 Ex7 | Fbxw7 Ex 9+10 | Notch HD-N1 | ||||||
| Prednisone response | Poor | Poor | Good | Good | Good | Good | Poor | Good |
| Final risk group | HR | HR | MR | MR | MR | SR | HR | SR |
| Blasts (BM) d15, % | 36 | 1 | Not done | 73 | 66 | 16 | 83 | 0 |
| MRD day 33 | 1E-2 | 1E-3 | 1E-3 | ND | 1E-2 | 1E-8 | 1E-1 | 1E-8 |
| MRD day 78 | 1E-1 | 1E-4 | 1E-4 | ND | 1E-4 | 1E-8 | 1E-4 | 1E-8 |
| Relapse | No | No | No | No | No | No | Yes | No |
| Death | No | Yes | No | Yes | No | No | Yes | No |
| Cause of death | N/A | Infection during chemotherapy | N/A | Infection during chemotherapy | N/A | N/A | Toxicity in relapse therapy | N/A |
| CD38 MFI‡ | 7.99 | 31.86 | 19.62 | 23.01 | 50.75 | 9.38 | 28.15 | 28.71 |
| CD38 mRNA‡ | 4.77 | 8.02 | 2.77 | 4.96 | 28.87 | 1.55 | 1.49 | 1.2 |
| Increased survival with DARA | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Increased survival with chemo+DARA | No | Yes | Yes | Yes | Yes | No | No | No |
| Parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . |
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, y | 10 | 8 | 16 | 7 | 8 | 13 | 2 | 16 |
| Sex | M | M | M | F | M | M | M | M |
| WBC count (initial), ×109/L | 687 | 303 | 188 | 127 | 166 | 202 | 672 | 76 |
| Blast (PB) initial, % | 80 | 94 | 89 | 94 | 74 | 80.5 | 96.5 | 58 |
| Blast (BM) initial, % | No data | 98 | 88 | 98 | 96 | 84.5 | No data | 92 |
| Immunophenotype | Cortical T | Cortical T | Cortical T | Cortical T | Cortical T | Cortical T | Pre-T | Cortical T |
| Karyotype | 46,XY, t(X;9)(q22;p12), del(6)(q24)[15] | No MP | No MP | 48,XX,+4,+8[15] | 47,XY,-5, del(6)(q23),+9, add(17)(q21), +mar[11] | 46,XY, t(1;14)(p32∼33;q11) [15] | 46,XY, t(1;6)(p34;q22), del(9)(p21)[12]/46,XY[3] | 46,XY, del(4)(q31), add(7)(q34), add(15)(q13), inc[3]/46,XY[12] |
| Transcription factors (quantitative PCR*) | TAL1, LMO2, LYL | LMO1 | LMO2, LYL, MYB | HOXA9, HOXA10, LMO2, LYL, MYB | TAL1, LMO2, LYL | TAL1, LMO2 | LMO2, LYL | TAL1 |
| Mutations† | None detected | Notch HD-N1 | Notch PEST 1 | PTEN Ex 7 | WT1 Ex7 | Notch PEST 1 | Stat5B Ex16 | Fbxw7 Ex 9+10 |
| WT1 Ex7 | Fbxw7 Ex 9+10 | Notch HD-N1 | ||||||
| Prednisone response | Poor | Poor | Good | Good | Good | Good | Poor | Good |
| Final risk group | HR | HR | MR | MR | MR | SR | HR | SR |
| Blasts (BM) d15, % | 36 | 1 | Not done | 73 | 66 | 16 | 83 | 0 |
| MRD day 33 | 1E-2 | 1E-3 | 1E-3 | ND | 1E-2 | 1E-8 | 1E-1 | 1E-8 |
| MRD day 78 | 1E-1 | 1E-4 | 1E-4 | ND | 1E-4 | 1E-8 | 1E-4 | 1E-8 |
| Relapse | No | No | No | No | No | No | Yes | No |
| Death | No | Yes | No | Yes | No | No | Yes | No |
| Cause of death | N/A | Infection during chemotherapy | N/A | Infection during chemotherapy | N/A | N/A | Toxicity in relapse therapy | N/A |
| CD38 MFI‡ | 7.99 | 31.86 | 19.62 | 23.01 | 50.75 | 9.38 | 28.15 | 28.71 |
| CD38 mRNA‡ | 4.77 | 8.02 | 2.77 | 4.96 | 28.87 | 1.55 | 1.49 | 1.2 |
| Increased survival with DARA | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Increased survival with chemo+DARA | No | Yes | Yes | Yes | Yes | No | No | No |
BM, bone marrow; F, female; Fbxw7, F-box and WD repeat domain containing 7; HOXA9/10, homeobox A9/10; HR, high risk; IL7R, interleukin-7 receptor; JAK1, janus kinase 2; LEF, lymphoid enhancer binding factor; LMO1/2, LIM domain only 1/2; LYL1, LYL1 basic helix-loop-helix family member; M, male; MEF2C, myocyte enhancer factor 2C; MR, medium risk; MRD, minimal residual disease; MYB, MYB proto-oncogene, transcription factor; N/A, not applicable; ND, not determined; no MP, no metaphases to analyze; Notch-HD N1, Notch N terminal heterodimerization domain; Notch PEST1, Notch proline, glutamic acid, serine, threonine–rich domain; PB, peripheral blood; PTEN, phosphatase and tensin homolog; SR, standard risk; STAT5B, signal transducer and activator of transcription 5B; TAL1, T-cell acute lymphocytic leukemia 1; TLX1/3, T-cell leukemia homeobox 1/3; WBC, white blood cell; WT1, WT1 transcription factor.
Relative expression values >10-fold beyond control (normal human T cells); see also supplemental Figure 3 and supplemental Methods. TLX1 and TLX3 were below the cutoff in all samples but borderline in patients 2 and 4. MEF2C was also tested, but all patients were negative.
Mutations in IL7R exon 6; JAK1 exons 15, 16, and 18; and LEF exons 2 and 3 were also tested but not detected.
Normalized to MFI/mRNA values in RPMI8226 multiple myeloma cells, respectively.
Combination of DARA and chemotherapy in vivo. (A) Eight randomly selected T-ALL PDX samples were injected into NSG mice via intrafemoral transpantation. Mice were left untreated (control) or treated with daratumumab (DARA), acute lymphoblastic leukemia-induction-like chemotherapy (chemo), or the combination (chemo+DARA). Survival was analyzed using the Kaplan-Meier method and log-rank statistics. P < .05 was considered statistically significant. (B) Minimal residual disease measured by PCR for patient-specific immunoglobulin/T-cell receptor rearrangements in bone marrow samples isolated from mice treated with DARA or chemo+DARA. *Low positive, not quantifiable. n. d., not determined. (C) CD38 MFI values as measured by flow cytometry. Expression levels were normalized to the RPMI8226 cell line. The dotted line shows the 25th percentile. Mice carrying patient samples 1 to 5 showed improved survival with DARA monotherapy (red bars), and mice with patient samples 6 to 8 did not (blue bars).
Combination of DARA and chemotherapy in vivo. (A) Eight randomly selected T-ALL PDX samples were injected into NSG mice via intrafemoral transpantation. Mice were left untreated (control) or treated with daratumumab (DARA), acute lymphoblastic leukemia-induction-like chemotherapy (chemo), or the combination (chemo+DARA). Survival was analyzed using the Kaplan-Meier method and log-rank statistics. P < .05 was considered statistically significant. (B) Minimal residual disease measured by PCR for patient-specific immunoglobulin/T-cell receptor rearrangements in bone marrow samples isolated from mice treated with DARA or chemo+DARA. *Low positive, not quantifiable. n. d., not determined. (C) CD38 MFI values as measured by flow cytometry. Expression levels were normalized to the RPMI8226 cell line. The dotted line shows the 25th percentile. Mice carrying patient samples 1 to 5 showed improved survival with DARA monotherapy (red bars), and mice with patient samples 6 to 8 did not (blue bars).
In order to assess depth of remission, we next performed polymerase chain reaction (PCR) for MRD in the bone marrow of 8 surviving xenograft mice (4/8 DARA and 4/8 chemo+DARA), as previously described.4 One set of mice (patient 4) was not analyzed, because MRD markers had not been established. Strikingly, 7 out of 8 mice were MRD negative in 2 markers (Figure 1B). One mouse in the DARA group (patient 1) showed a low positive, not quantifiable signal in 1 marker (Figure 1B).6 The mouse from the same patient in the chemo+DARA group was MRD positive, and flow cytometry also revealed leukemic infiltration in bone marrow and spleen (Figure 1B and data not shown). In order to evaluate a potential connection between CD38 expression and preclinical response to DARA, mean fluorescence intensity (MFI) of CD38 surface expression and the CD38 messenger RNA (mRNA) level were determined in all samples with reference to the RPMI8226 multiple myeloma cell line. Our data do not show a conclusive link between CD38 surface expression and DARA response (Figure 1C and Table 1). Of 5 samples showing improved survival with DARA monotherapy, 4 samples (80%) had a CD38 surface expression above the 25th percentile (Figure 1C; supplemental Figure 2). Likewise, of 3 samples that did not show improved survival with DARA monotherapy, 2 samples (67%) had a comparatively high CD38 surface expression above the 25th percentile (Figure 1C; supplemental Figure 2). Patient 1 with a CD38 expression below the 25th percentile showed improved survival with DARA, but not with chemo+DARA, which remains unexplained. Improved survival with DARA was independent of genetic subgroups (Table 1; supplemental Figure 3).
Our results indicate that DARA may be a potent option in T-ALL warranting further clinical studies. Our data show that DARA alone causes MRD negativity in a substantial proportion of T-ALL PDX mice responding to the drug in a phase 2-like setting. Also, it is important to note that induction-like chemotherapy with vincristine, dexamethasone, and pegylated Escherichia coli asparaginase, all substances with particular risks and side effects, does not add an overall survival advantage in our experiments. This is of particular relevance for patients with refractory or relapsed disease with limited bone marrow reserves and a high likelihood of chemotherapy-related complications. On the other hand, the addition of chemotherapy may delay the outgrowth of T-ALL samples and may for that reason still be valuable in combination with DARA. The observation that patient 1 showed enhanced survival with DARA, but not with chemo+DARA, may be explained by potential toxic effects of chemotherapy drugs that may interfere with immunological effector mechanisms triggered by a therapeutic antibody. It remains unclear whether antigen escape is a problem in CD38-targeted therapy similarly to what is observed when targeting CD19. CD38 expression usually remains stable over the course of T-ALL, and CD38-negative relapses have only anecdotally been described in multiple myeloma.7 In our experiment, all post-DARA samples were CD38 positive, and we have no evidence for antigen escape (data not shown). DARA is a native unmodified immunoglobulin G1 antibody exerting different effector functions in vitro.8 The antibody has been shown to kill tumor cells by antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity.8,9 Antibody-dependent cell-mediated cytotoxicity mediated by lymphocytes and complement-dependent cytotoxicity cannot be assessed in NSG mice and require more sophisticated models. However, the therapeutic effects observed here may be due to macrophage activity and presumably antibody-dependent cellular phagocytosis, similar to our descriptions for CD19-targeting antibodies,4,10 or the activation of other myeloid effector cells such as granulocytes. In addition, DARA interferes with CD38 ectoenzyme activity and induces direct cell death upon Fcγ receptor–mediated CD38 crosslinking,11 suggesting that induction of direct cell death may be an additional and effector-cell–independent mechanism. Also, modulation of CD38 in a patient’s own immune cells by DARA (eg, elimination of a leukemia-protective immunosuppressive microenvironment consisting of regulatory T cells, B cells, and myeloid-derived suppressor cells) may be relevant.12,13 However, the fact that DARA works well in PDX models in NSG mice supports the contribution of myeloid effector cells such as macrophages and warrants antibody engineering strategies focusing on a better recruitment of these effector populations. All in all, DARA may be a promising novel therapeutic option in T-ALL.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Joachim Kunz for critical reading of the manuscript. The authors also thank Katrin Timm-Richert, Katrin Neumann, Martina Kähler, Margit Happich, and Maike Hagedorn for excellent technical assistance.
This work was supported by the Deutsche Krebshilfe (grant 70113524) (D.M.S. and C.K.) and the Deutsche José-Carreras Leukämiestiftung (grant DJCLS 17 R/2017) (D.M.S. and C.K.). M.P. is supported by the Deutsche Krebshilfe Mildred-Scheel professorship program. A.K.B. is supported by the Deutsche Forschungsgemeinschaft (BE 6555/1-1).
Authorship
Contribution: F.V. designed and performed experiments and analyzed data; D.W., L.L., and S.B. performed experiments and analyzed data; G.C., T.V., and M.S. contributed patient material; P.R.-P., A.E.K., J.L., and A.K.B. contributed genetic data; F.-S.F. performed statistical analyses; M.P., T.V., C.K., and D.M.S. supervised the research direction; F.V. and D.M.S. wrote the manuscript; and all authors discussed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denis M. Schewe, Department of Pediatrics I, University Medical Center Schleswig-Holstein, Arnold-Heller-Str 3, 24105 Kiel, Germany; e-mail: denis.schewe@uksh.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal