TO THE EDITOR:
Vitreoretinal lymphoma (VRL) is a rare ocular malignancy that occurs as a primary intraocular lymphoma or secondary manifestation of primary central nervous system lymphoma (PCNSL).1 Although the diagnosis is classically made by cytologic and immunohistochemical confirmation of malignant B cells, molecular analyses of vitreous aspirates are increasingly being used. The main challenge to VRL diagnosis is tumor-cell fragility, leading to paucicellular samples that preclude routine analysis. A high frequency of MYD88 mutations has been reported in diffuse large B-cell lymphomas (DLBCLs), including VRL and PCNSL. In these lymphomas, an MYD88L265P nonsynonymous point mutation, resulting in an amino acid substitution of proline (CCG) for leucine (CTG) at position 265, is the most common mutation, accounting for >60% of all mutations.2-5 This finding has prompted many research groups to incorporate MYD88L265P mutational screening into VRL diagnostics.5-10 However, conventional polymerase chain reaction (PCR) and Sanger sequencing–based MYD88 mutational detection have a low sensitivity of 25%.11 Allele-specific PCR coupled with high-resolution melting curve analysis has a modestly improved sensitivity, by a further 5%,9 but this method is restricted to determining the ratio of MYD88WT/MYD88L265P alleles in bulk-sample analyses and does not reflect the composition of cellular heterogeneity. An approach that confers higher detection sensitivity is urgently needed for VRL diagnosis, where low cellularity in the vitreous body aspirate can lead to false-positive results, limiting the use of B-cell clonality assessment for VRL diagnosis.9
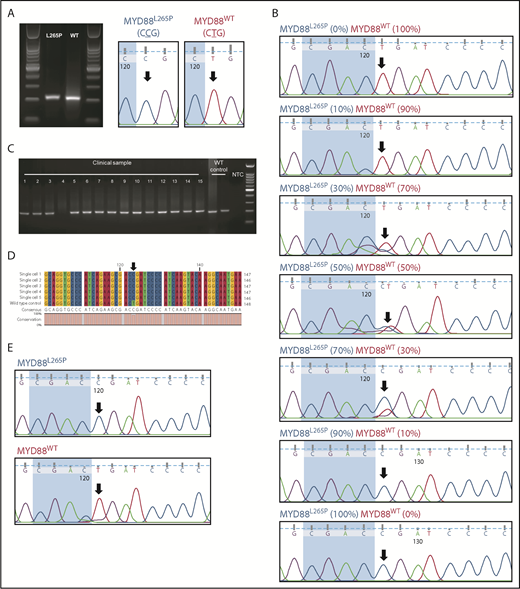
To illustrate the low detection sensitivity of Sanger sequencing in bulk samples, we conducted PCR to isolate both wild-type MYD88WT and mutant MYD88L265P alleles and mixed them in various proportions (amounting to 100 ng) before Sanger sequencing (Figure 1A). When the reaction consisted of 100% MYD88WT amplicon (100-ng equivalent), we observed a single (red) peak on the electropherogram corresponding to the thymidine of the wild-type MYD88WT allele (Figure 1B). Similarly, we observed a single (blue) peak corresponding to the mutant allele when 100% of the MYD88L265P amplicon was added to the reaction (Figure 1B). When both amplicons were mixed in equal proportions (50 ng each), we observed a double (red/blue) peak on the electropherogram, indicating equal representation of the cytosine (MYD88L265P) and thymidine (MYD88WT) nucleosides (Figure 1B). The detection limit of Sanger sequencing for both MYD88WT and MYD88L265P at their lowest proportions was 30% (Figure 1B).
Sanger sequencing detection limit and single-cell MYD88 PCR analysis. (A) MYD88 PCR was conducted to amplify both MYD88L265P and MYD88WT alleles. Amplified sequences were verified to encode either CCG or CTG corresponding to the mutant MYD88L265P and MYD88WT alleles, respectively. (B) The PCR amplicons were mixed in titrating proportions to determine the Sanger sequencing detection limit. The lowest detection limit of Sanger sequencing in detecting MYD88L265P and MYD88WT MYD88 alleles was ∼30%. (C) Single-cell MYD88 PCR was conducted on a clinical DLBCL sample with 15 single cells (1-15); 14 of 15 cells gave a PCR band of ∼200 bp. (D) Multiple sequence alignment of the excised and sequenced PCR amplicon showed a T → C point mutation in 5 representative cells from clinical samples but not in Pfeiffer cells (a germinal center B cell–like DLBCL exhibiting the MYD88WT allele). (E) Electropherogram showed clean and well-resolved sequencing peaks for MYD88L265P and MYD88WT. The black arrows throughout indicate the location of the point mutation. NTC, no template control; WT, wild type.
Sanger sequencing detection limit and single-cell MYD88 PCR analysis. (A) MYD88 PCR was conducted to amplify both MYD88L265P and MYD88WT alleles. Amplified sequences were verified to encode either CCG or CTG corresponding to the mutant MYD88L265P and MYD88WT alleles, respectively. (B) The PCR amplicons were mixed in titrating proportions to determine the Sanger sequencing detection limit. The lowest detection limit of Sanger sequencing in detecting MYD88L265P and MYD88WT MYD88 alleles was ∼30%. (C) Single-cell MYD88 PCR was conducted on a clinical DLBCL sample with 15 single cells (1-15); 14 of 15 cells gave a PCR band of ∼200 bp. (D) Multiple sequence alignment of the excised and sequenced PCR amplicon showed a T → C point mutation in 5 representative cells from clinical samples but not in Pfeiffer cells (a germinal center B cell–like DLBCL exhibiting the MYD88WT allele). (E) Electropherogram showed clean and well-resolved sequencing peaks for MYD88L265P and MYD88WT. The black arrows throughout indicate the location of the point mutation. NTC, no template control; WT, wild type.
To overcome the low detection sensitivity of Sanger sequencing in MYD88L265P mutational detection, we established a single-cell diagnostic workflow using the DEPArray NxT system (Menarini Silicon Biosystems) coupled with Sanger sequencing to determine the MYD88 mutational profile of single B cells isolated from the vitreous fluid (supplemental Figure 1A, available on the Blood Web site). Because a majority (∼95%) of VRLs are of B-cell origin, we focused on cytologically proven DLBCL to validate the feasibility of our approach (supplemental Figure 2). Here, single B cells were isolated based on a staining panel (CD3, CD19, CD20, and CD79a) to facilitate B-cell isolation, even in the event of CD20 downregulation in rituximab-treated patients.
Next, we conducted whole-genome amplification on isolated single CD19+CD20+ B cells to increase the amount of DNA for single-cell MYD88 PCR (supplemental Methods). Amplicons corresponding to 200 bp were observed in 14 of 15 single cells (Figure 1C) isolated from a patient with DLBCL (Table 1 patient 1a; supplemental Figure 2). The 200-bp amplicon was absent in the no template negative control. By multiple sequence alignment (Figure 1D), we found 5 representative cells with 100% sequence alignment with the CCG point mutation (MYD88L265P); this mutation was not detected in the MYD88WT control Pfeiffer cells (CTG). Our approach thus demonstrates that single-cell isolation followed by single-cell MYD88 PCR and Sanger sequencing can generate an unambiguous DNA trace to determine MYD88L265P mutational status (Figure 1E).
Single-cell MYD88 sequencing of CD19+CD20+ B cells isolated from patients with DLBCL or chronic inflammation
| Patient . | Clinical diagnosis . | MYD88WT . | MYD88L265P homozygous . | MYD88L265P heterozygous . |
|---|---|---|---|---|
| 1* | DLBCL | |||
| a | Initial diagnosis | 0 | 14 | 0 |
| b | Disease recurrence | 3 | 2 | 4 |
| 2† | DLBCL | 1 | 4 | 4 |
| 3 | DLBCL | 5 | 2 | 3 |
| 4 | Inflammation | 5 | 0 | 0 |
| 5 | Inflammation | 0 | 1 | 21 |
| 6 | Inflammation | 0 | 0 | 13 |
| Patient . | Clinical diagnosis . | MYD88WT . | MYD88L265P homozygous . | MYD88L265P heterozygous . |
|---|---|---|---|---|
| 1* | DLBCL | |||
| a | Initial diagnosis | 0 | 14 | 0 |
| b | Disease recurrence | 3 | 2 | 4 |
| 2† | DLBCL | 1 | 4 | 4 |
| 3 | DLBCL | 5 | 2 | 3 |
| 4 | Inflammation | 5 | 0 | 0 |
| 5 | Inflammation | 0 | 1 | 21 |
| 6 | Inflammation | 0 | 0 | 13 |
1a and 1b are samples from the same patient, before and after (12 mo) intravitreal rituximab and methotrexate therapy, taken during initial diagnosis and disease recurrence, respectively.
Consisted of single cells isolated from both eyes and the aqueous humor.
Our single-cell approach allowed us to further characterize CD19+CD20+ single B cells in patients with VRL or chronic inflammation and classify patients based on the composition of these single cells as MYD88WT, MYD88L265P heterozygous, or MYD88L265P homozygous (Table 1). We found that patients with clinically and cytologically confirmed VRL exhibited a mixed zygosity signature that contained MYD88L265P homozygous mutations (Table 1 patients 1-3). Patients with confirmed chronic inflammation, negative VRL cytology, and no immunoglobulin H (IgH) rearrangement clonality exhibited a zygosity signature that predominantly consisted of both MYD88WT and MYD88L265P heterozygous mutations (Table 1 patients 4-6). Patient 1 with confirmed VRL was treated with intravitreal rituximab and methotrexate therapy and was in remission until 1 year later. Post-therapy analysis of ocular fluid with recurrence showed an increase in MYD88L265P heterozygous and MYD88WT alleles and a decrease in MYD88L265P homozygous alleles (Table 1 patient 1a-b), thus shifting toward a chronic inflammation profile. Interestingly, we found 1 MYD88L265P homozygous mutant cell in patient 5 and a high proportion of MYD88L265P heterozygous mutant cells in patients 5 and 6 with chronic inflammation (Table 1 patients 4-6). Because recurrent MYD88L265P somatic mutations have been reported in B-cell lymphomas, different zygosity profiles and shifts with therapy may reflect ongoing somatic mutations at different time points of disease.12
Recent studies of Waldenström macroglobulinemia and other B-cell lymphomas, such as IgM monoclonal gammopathy of undetermined significance (IgM-MGUS), have suggested a role for MYD88 zygosity in prognosticating the therapeutic response and disease risk.13-15 In Waldenström macroglobulinemia, Treon et al13,15 reported patients with wild-type MYD88 to be associated with decreased treatment response and worse prognosis; however, in IgM-MGUS, Varettoni et al14 reported that patients with MYD88L265P mutations had a greater disease burden and higher risk of disease progression. On the basis of these findings, we hypothesize that the presence of homozygous and heterozygous MYD88L265P mutations in our patients with chronic inflammation, similar to IgM-MGUS, might represent a greater disease burden and thus higher risk for disease progression. Although the zygosity shift posttherapy in patient 1 may represent a lower disease burden, the continued presence of MYD88L265P heterozygosity still reflects ongoing, albeit less aggressive, disease. Both these observations require further validation beyond the scope of this current work but represent our next steps for research.
In summary, our approach has enabled us to dissect and resolve MYD88L265P mutation heterogeneity at the single-cell resolution, which is otherwise unattainable with allelic-specific PCR or high-resolution melting curve analysis.16 Notably, although the current technology can enumerate the copy numbers of MYD88L265P mutations in bulk-sample analysis,7,11,17 single-cell analysis elucidates sample composition, differentiating MYD88L265P heterozygous mutations from MYD88L265P homozygous mutations. In short, such single-cell zygosity profiling allows us to avoid false-positive/false-negative outcomes from conventional PCR analysis by providing the complete MYD88 mutational profile. Although whole-exome sequencing and single-nucleotide polymorphism microarray can provide sequencing data with greater coverage and depth, we propose that our method is more cost and time efficient.18,19
Single-cell MYD88 mutational analysis goes beyond dissecting the dichotomous outcomes of VRL diagnosis; it has potential utility in VRL and PCNSL disease monitoring, where MYD88 zygosity composition can be profiled in vitreous and cerebrospinal fluid before and during treatment. Given the role of the MYD88L265P mutation in other B-cell lymphomas,5-8,20 the use of single-cell MYD88 sequencing also has wider application beyond zygosity profiling in VRL. Furthermore, our workflow could also be employed for single T-cell analysis where the detection of VRL with T-cell lymphoma manifestation is warranted (supplemental Figure 1B). Although our workflow permits the selection of CD3-expressing T cells, it should be noted that the MYD88 mutation, which is specific for DLBCL, would be less applicable to T-cell lymphoma detection. Nonetheless, with the various driver mutations in T-cell lymphoma, as reported by Dong et al,21 the workflow we describe here could be employed for T-cell lymphoma screening in a similar manner.
In our study, we used the DEPArray system to identify and sort individual rare target B cells from vitreous biopsies to perform single cell–based MYD88 mutational profiling to aid VRL diagnosis. To date, most of the mainstream single-cell sorting instruments are microfluidics-based technologies (eg, fluorescence-activated cell sorting, 10× Genomics, and Drop-Seq) that require samples with high cell yields for phenotyping and molecular measurements of individual cells. However, the number of cells typically available from vitreous biopsies used for diagnosis is limited and heterogeneous. The ability to select, sort, and recover rare target malignant cells from vitreous biopsies poses a unique challenge for most microfluidics-based single-cell technologies. By contrast, the real-time imaging–based digital cell sorting DEPArray technology enables automated isolation of rare single B cells from paucicellular vitreous biopsies with the high resolution and purity required for sensitive downstream analyses, such as single cell–based MYD88 mutational profiling. At the moment, the DEPArray-based workflow needs to be performed in specialized laboratories; however, we believe that the unmet clinical needs for single cell–based precision diagnostics will precipitate the incorporation of this system into standard pathology laboratories in the near future. Furthermore, with the ease of use and automation that the DEPArray technology provides, laboratory technicians can be readily trained to operate the system.
The online version of this article contains a data supplement.
The data presented here are part of a patent filed on 21 August 2018 (#10201807097T).
Acknowledgments
The authors thank Leonard Tan, Department of Pathology, Singapore General Hospital, for his expert opinion in lymphoma and Insight Editing London for editing the manuscript before submission.
This study was wholly supported by research funding from the research collaboration between A. Menarini Biomarkers Singapore Pte Ltd, VisionSave, and the Singapore Eye Research Institute.
Authorship
Contribution: T.S.L., A.S.Y.C., and P.R.-C. conceived and designed the experiments; M.M.W. and W.J.T. performed the experiments; A.S.Y.C., T.T., and S.P.C. provided clinical material and data; W.J.T. wrote the manuscript; and all authors reviewed and approved the submission of manuscript.
Conflict-of-interest disclosure: W.J.T. and T.S.L. are researchers and P.R.-C. is a consultant for A. Menarini Biomarkers Singapore Pte Ltd. A.S.Y.C. received research funding from A. Menarini Biomarkers Singapore Pte Ltd. The remaining authors declare no competing financial interests.
Correspondence: Tong Seng Lim, A. Menarini Biomarkers Singapore Pte Ltd, 30 Pasir Panjang Rd, 08-32 Mapletree Business City, Singapore 117440; e-mail: tongseng.lim@mbiomarkers.com; and Anita Sook Yee Chan, Singapore National Eye Centre and Singapore Eye Research Institute, The Academia, 20 College Rd, Discovery Tower Level 6, Singapore 169856; e-mail: anita.chan.s.y@singhealth.com.sg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal