Key Points
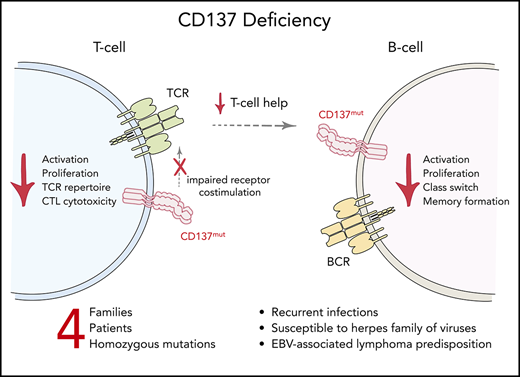
CD137 deficiency is a novel inborn error of immunity with immune dysregulation and EBV-associated lymphomagenesis.
Our study highlights the key role of CD137 for immune homeostasis with relevance to immunodeficiency and cancer immunotherapy.
Abstract
Dysregulated immune responses are essential underlying causes of a plethora of pathologies including cancer, autoimmunity, and immunodeficiency. We here investigated 4 patients from unrelated families presenting with immunodeficiency, autoimmunity, and malignancy. We identified 4 distinct homozygous mutations in TNFRSF9 encoding the tumor necrosis factor receptor superfamily member CD137/4-1BB, leading to reduced, or loss of, protein expression. Lymphocytic responses crucial for immune surveillance, including activation, proliferation, and differentiation, were impaired. Genetic reconstitution of CD137 reversed these defects. CD137 deficiency is a novel inborn error of human immunity characterized by lymphocytic defects with early-onset Epstein-Barr virus (EBV)-associated lymphoma. Our findings elucidate a functional role and relevance of CD137 in human immune homeostasis and antitumor responses.
Introduction
Antigen receptors and associated signaling machineries function to sense and rapidly react to threats that endanger the organisms such as foreign invaders or altered self-structures. Coreceptors play fundamental roles in regulating and fine-tuning the signal strength of antigen receptors. Defective function of these immune receptors may lead to elevated susceptibility to infections, autoimmune manifestations, and cancer.1 Epstein-Barr virus (EBV) is one of the most prevalent viruses that infects humans and maintains lifelong latency.2,3 In individuals with impaired T-cell immunity, EBV infection may result in lymphoproliferative disease, or malignant lymphomas of T-, B-, or natural killer (NK)-cell origin.3 To date, germline genetic mutations affecting CD27, PRKCD, RASGRP1, MAGT1, SH2D1A, ITK, and others have been identified to cause immune dysregulation with EBV-associated diseases.2 Studies of these patients have provided mechanistic insight into pathways required for robust host immune surveillance against EBV infection and associated lymphomas. Here, we report an inborn deficiency of tumor necrosis factor (TNF) receptor superfamily member 9 (TNFRSF9)/CD137/4-1BB with marked immune dysregulation and predisposition to EBV-associated lymphoma.
Study design
Patients
The study was approved by the institutional review boards of the Medical University of Vienna (Vienna, Austria; EK499/2011), the Ludwig Maximilian University of Munich (Munich, Germany), Sheba Medical Center (Tel HaShomer, Israel), and the Baylor University College of Medicine (Houston, TX). All study participants provided written informed consent. Informed assent was obtained for children.
Experimental methodologies
All methods are detailed in the supplemental Materials and methods (available on the Blood Web site).
Results and discussion
Clinical phenotypes
We studied 4 patients from 4 unrelated families, 3 of whom were from consanguineous background (Figure 1A). Patients had early childhood recurrent infections of bacterial and viral origin, and signs of autoimmunity (Figure 1B-C; supplemental Figure 1; supplemental Table 1). Sinopulmonary and herpes virus infections were common. All patients exhibited hepatomegaly, splenomegaly, and/or lymphadenopathy. Signs of autoimmunity, including hemolytic anemia, were present in some patients. We found abnormal immunoglobulin levels in all patients (supplemental Table 2). Patients 1 and 3 developed EBV-related B-cell lymphoma, patient 2 had an autoimmune lymphoproliferative syndrome–like phenotype, and patient 4 was diagnosed as having common variable immune deficiency. Therapeutic regimens included chemotherapy, immunosuppression, antibiotic prophylaxis, and regular immunoglobulin substitution (supplemental Table 1).
Genetic and clinical presentation of 4 patients with immune dysregulation. (A) Pedigrees showing 4 families with affected individuals (patients) harboring TNFRSF9 mutations. Solid symbols indicate affected persons who were homozygous for the mutant allele; half-solid symbols, heterozygous persons; center solid symbols, unaffected persons homozygous for the mutant allele; circles, female family members; squares, male family members; double lines, consanguinity. (B) Radiographic features in patients with CD137 deficiency. Top left, Coronal T2-HASTE MR demonstrating a lobulated mass of the small intestine (white arrow) in patient 1 (P1). Top right, axial T2-HASTE STIR MR image of upper abdomen depicting hyperintense multiple metastatic liver nodules (red arrow) and bilateral renal cortical hypointense metastatic nodules (blue arrow) in patient 1. Bottom left, Chest computer tomography (CT) scan indicating right-sided hilar lymph nodes in patient 3 (P3). Bottom right, chest CT scan revealing bilateral ground-glass opacities in patient 2 (P2). (C) Top left, sections of right inguinal lymph node tissue (hematoxylin-and-eosin staining) showing numerous small regressive germinal centers (black arrow) in patient 2 (scale bar, 10 µm). Bottom right, Ki-67 immunostain in patient 2, positive in 20% to 30% of the cells exhibiting a moderate lymphocyte proliferative activity (scale bar, 10 µm). Bottom left, in situ hybridization for EBV-encoded small RNAs (EBER) displaying numerous positive cells in blue in patient 3 (scale bar, 200 µm). Top right, Multinucleated Reed-Sternberg cells (black arrows) typical of Hodgkin lymphoma (scale bar, 20 µm) in a resection of a submandibular lymph node in patient 3. (D) Schematic illustration displaying the interaction of ligands expressed on antigen-presenting cells (APCs) with their respective receptors on activated T cells, including the interaction between CD137L and CD137. (E) Localization of CD137 mutations in our patients. CD137 gene and protein domains with the 4 newly identified mutations are indicated. (F) CD137 protein expressions in activated (CD25+) CD4+ and CD8+ T cells, activated (CD86+) CD19+ B cells, and activated (interleukin 2 [IL-2] stimulated) NK cells (CD56+) in patients and healthy donors (HDs) demonstrating complete loss or markedly reduced expression in patients’ cells, measured by flow cytometry. All error bars indicate plus or minus standard error of mean (SEM). CRD, cysteine-rich domain; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; P4, patient 4; UTR, untranslated region.
Genetic and clinical presentation of 4 patients with immune dysregulation. (A) Pedigrees showing 4 families with affected individuals (patients) harboring TNFRSF9 mutations. Solid symbols indicate affected persons who were homozygous for the mutant allele; half-solid symbols, heterozygous persons; center solid symbols, unaffected persons homozygous for the mutant allele; circles, female family members; squares, male family members; double lines, consanguinity. (B) Radiographic features in patients with CD137 deficiency. Top left, Coronal T2-HASTE MR demonstrating a lobulated mass of the small intestine (white arrow) in patient 1 (P1). Top right, axial T2-HASTE STIR MR image of upper abdomen depicting hyperintense multiple metastatic liver nodules (red arrow) and bilateral renal cortical hypointense metastatic nodules (blue arrow) in patient 1. Bottom left, Chest computer tomography (CT) scan indicating right-sided hilar lymph nodes in patient 3 (P3). Bottom right, chest CT scan revealing bilateral ground-glass opacities in patient 2 (P2). (C) Top left, sections of right inguinal lymph node tissue (hematoxylin-and-eosin staining) showing numerous small regressive germinal centers (black arrow) in patient 2 (scale bar, 10 µm). Bottom right, Ki-67 immunostain in patient 2, positive in 20% to 30% of the cells exhibiting a moderate lymphocyte proliferative activity (scale bar, 10 µm). Bottom left, in situ hybridization for EBV-encoded small RNAs (EBER) displaying numerous positive cells in blue in patient 3 (scale bar, 200 µm). Top right, Multinucleated Reed-Sternberg cells (black arrows) typical of Hodgkin lymphoma (scale bar, 20 µm) in a resection of a submandibular lymph node in patient 3. (D) Schematic illustration displaying the interaction of ligands expressed on antigen-presenting cells (APCs) with their respective receptors on activated T cells, including the interaction between CD137L and CD137. (E) Localization of CD137 mutations in our patients. CD137 gene and protein domains with the 4 newly identified mutations are indicated. (F) CD137 protein expressions in activated (CD25+) CD4+ and CD8+ T cells, activated (CD86+) CD19+ B cells, and activated (interleukin 2 [IL-2] stimulated) NK cells (CD56+) in patients and healthy donors (HDs) demonstrating complete loss or markedly reduced expression in patients’ cells, measured by flow cytometry. All error bars indicate plus or minus standard error of mean (SEM). CRD, cysteine-rich domain; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; P4, patient 4; UTR, untranslated region.
Genetic evaluation and loss-of-function mutations in TNFRSF9
To elucidate the disease etiology, we performed whole-exome sequencing and identified distinct homozygous variants in TNFRSF9 encoding the costimulatory immune checkpoint CD137/4-1BB (Figure 1D-E; supplemental Figure 2; supplemental Table 3). Parents were heterozygous carriers in all cases. All TNFRSF9 variants were absent in gnomAD, and predicted to be deleterious using common prediction algorithms (supplemental Table 4). Patient 1 was homozygous for a large deletion in TNFRSF9, and patient 2 harbored a homozygous missense mutation affecting evolutionarily conserved residues (supplemental Figure 3). Patient 3 was homozygous for a TNFRSF9 mutation disrupting the splice-acceptor site of exon 3, resulting in the skipping of exons 3 and 6. It remains unclear why alternative splice variants are present in this patient, including a smaller fraction of transcripts that has both exon 3 and exon 6 of TNFRSF9 skipped (supplemental Figure 4). However, it is possible that this specific splice-acceptor site regulates the splicing of nearby exons, as demonstrated by variants in ERBB4.4 Additional investigations in the splicing effects of this genetic variant were, however, beyond the scope of this study. Patient 4 was homozygous for a mutation in the splice-donor site of exon 2 causing skipping of exon 2 (supplemental Figure 4). All mutations resulted in markedly reduced or abrogated expression of CD137 on activated T, B, and NK cells, indicating a loss-of-function phenotype (Figure 1F; supplemental Figure 5). However, the loss of CD137 did not affect CD137L protein expression in patients’ T cells (supplemental Figure 7D). Recent studies showed that CD137 can be transferred from Hodgkin and Reed-Sternberg cells to neighboring cells by trogocytosis.5 We thus investigated the expression of CD137L in T cells by reverse transcription polymerase chain reaction and found that TNFSF9/CD137L messenger RNA is expressed intrinsically in T cells (supplemental Figure 7D).
Interestingly, in 3 of the pedigrees, 1 healthy sibling each was also homozygous for the same TNFRSF9 mutation. Accordingly, they had abrogated or reduced CD137 expression, without overt clinical disease. It is currently unknown whether CD137 deficiency may be aggravated by infections or other extrinsic challenges. Incomplete penetrance is well known for pathogenic immune system mutations,6 especially for defects with predominant immune dysregulation, as exemplified by CTLA-4 haploinsufficiency.7
Immune-cell phenotypes
Patients had variable lymphocyte abnormalities (supplemental Table 2). All patients had elevated proportions of transitional and immature B cells but markedly reduced memory B cells and plasmablasts. Decreased NK-cell counts were observed in patients 2 and 3. Patients 1, 2, and 3 had reduced follicular helper T cells (TFH) (Figure 2A; supplemental Figure 6).
Immunological and functional phenotypes in CD137-deficient patients. (A) T- and B-cell features in our patients: immunophenotyping revealed decreased frequencies of class-switched (CD27+ immunoglobulin D–negative [IgD−]) B cells and TFH (CD45RO+CXCR5+) cells in patient 1 (P1) compared with an HD, as measured by flow cytometry. (B) T-cell proliferation with carboxyfluorescein succinimidyl ester (CFSE) fluorescence cell incorporation assay 4 days poststimulation exhibiting reduced CD3+ T-cell proliferation in response to anti-CD3 and anti-CD3 in combination with CD137L in patients 1, 2 (P2), and 4 (P4) with partial and complete restoration upon OX40 and CD28 costimulation, respectively (****P < .0001; 2-way analysis of variance [ANOVA]). (C) Representative surface expression of CD25 on T cells as measured by flow cytometry 4 days poststimulation in patient 2 compared with an HD, demonstrating a T-cell activation defect with a compensatory effect upon CD28 costimulation. (D) Rescue of T-cell proliferation and activation via CD25 expression in patient 3 (P3) by exogenous expression of wild-type CD137. (E) Analysis of T-cell receptor γ (TRG) repertoire diversity with a tree-map representation for patients 1, 2, and 3, and age-matched healthy controls. Each colored square represents a unique clone and its size reflects its productive frequency within the repertoire. Simpson's D diversity index and Shannon's H index quantify repertoire clonality. (F) Flow cytometric expression of Tregs, displaying reduced Treg rates in patients 1 and 2 compared with an HD. (G) Top, Flow cytometric expression of CD86+ and CD25+ of CD19+CD3− cells 1 day poststimulation with CD40L in combination with IL4 showed impaired activation in patients 2 and 3. Bottom, quantification of B-cell activation, showing significantly lower B-cell activation in patient cells compared with HDs (*P < .05; ***P < .001; 2-way ANOVA). (H) Top, class-switched IgG+ and IgA+ of CD19+ cells upon various stimulations, displayed impaired class switch recombination in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, Quantification of class-switched (CD27+IgD−) CD19+ cell rates showing significantly lower frequencies in the patients (**P < .01; ***P < .001; 2-way ANOVA). (I) Top, B-cell proliferation measured by violet proliferation dye (VPD450) 4 days poststimulation showing reduced proliferating B cells in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, quantification of CD19+ proliferating cells, showing decreased rates in patients (*P <.05; 2-way ANOVA). All error bars indicate plus or minus SEM. CDR, complementarity-determining region; CpG, cytosine guanine dinucleotide; IGH, immunoglobulin heavy; Unstim, unstimulated.
Immunological and functional phenotypes in CD137-deficient patients. (A) T- and B-cell features in our patients: immunophenotyping revealed decreased frequencies of class-switched (CD27+ immunoglobulin D–negative [IgD−]) B cells and TFH (CD45RO+CXCR5+) cells in patient 1 (P1) compared with an HD, as measured by flow cytometry. (B) T-cell proliferation with carboxyfluorescein succinimidyl ester (CFSE) fluorescence cell incorporation assay 4 days poststimulation exhibiting reduced CD3+ T-cell proliferation in response to anti-CD3 and anti-CD3 in combination with CD137L in patients 1, 2 (P2), and 4 (P4) with partial and complete restoration upon OX40 and CD28 costimulation, respectively (****P < .0001; 2-way analysis of variance [ANOVA]). (C) Representative surface expression of CD25 on T cells as measured by flow cytometry 4 days poststimulation in patient 2 compared with an HD, demonstrating a T-cell activation defect with a compensatory effect upon CD28 costimulation. (D) Rescue of T-cell proliferation and activation via CD25 expression in patient 3 (P3) by exogenous expression of wild-type CD137. (E) Analysis of T-cell receptor γ (TRG) repertoire diversity with a tree-map representation for patients 1, 2, and 3, and age-matched healthy controls. Each colored square represents a unique clone and its size reflects its productive frequency within the repertoire. Simpson's D diversity index and Shannon's H index quantify repertoire clonality. (F) Flow cytometric expression of Tregs, displaying reduced Treg rates in patients 1 and 2 compared with an HD. (G) Top, Flow cytometric expression of CD86+ and CD25+ of CD19+CD3− cells 1 day poststimulation with CD40L in combination with IL4 showed impaired activation in patients 2 and 3. Bottom, quantification of B-cell activation, showing significantly lower B-cell activation in patient cells compared with HDs (*P < .05; ***P < .001; 2-way ANOVA). (H) Top, class-switched IgG+ and IgA+ of CD19+ cells upon various stimulations, displayed impaired class switch recombination in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, Quantification of class-switched (CD27+IgD−) CD19+ cell rates showing significantly lower frequencies in the patients (**P < .01; ***P < .001; 2-way ANOVA). (I) Top, B-cell proliferation measured by violet proliferation dye (VPD450) 4 days poststimulation showing reduced proliferating B cells in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, quantification of CD19+ proliferating cells, showing decreased rates in patients (*P <.05; 2-way ANOVA). All error bars indicate plus or minus SEM. CDR, complementarity-determining region; CpG, cytosine guanine dinucleotide; IGH, immunoglobulin heavy; Unstim, unstimulated.
Functional T-cell defects in CD137-deficient patients
Cd137-deficient mice have impaired T-cell survival, proliferation, and cytotoxicity.8-10 We thus hypothesized that human CD137 deficiency may also hamper T-cell differentiation and function. Indeed, T-cell proliferation responses to various stimuli were reduced in all patients (Figure 2B). Surprisingly, patients’ T cells showed impaired proliferation to anti-CD3 stimulation alone. We hypothesized that CD137L is functionally important and required to elicit a normal cellular response to CD3. By blocking CD137 in healthy donor (HD) peripheral blood mononuclear cells (PBMCs) stimulated with anti-CD3, we showed a dose-dependent reduction in T-cell proliferation (supplemental Figure 7A). Indeed, CD137 receptor/ligand interaction plays a functional role in T cells. Remarkably, the addition of anti-CD28–mediating T-cell receptor (TCR) costimulation restored proliferative functions, and partial compensation was seen upon OX40 costimulation (Figure 2B). We also observed reduced T-cell activation in patients 1 to 3, amenable to correction upon additional CD28 costimulation. Patient 4 had normal T-cell activation (Figure 2C; supplemental Figure 7B), consistent with a milder T-cell proliferation defect (Figure 2B). Collectively, our findings highlight the importance of CD137 in immune homeostasis costimulation and, intriguingly, the lack of CD137 costimulation may be compensated in the presence of other costimulators.
To prove the causative role of lack of CD137 for the observed phenotypes, we performed a gene-rescue experiment in T cells from patient 3. Upon exogeneous expression of wild-type CD137, T-cell proliferation and activation defects were restored (Figure 2D; supplemental Figure 7C).
TCR repertoire analysis in patients 1 to 3 showed significant clonal expansion associated with reduced diversity (Figure 2E). CD137 is expressed in regulatory T cells (Tregs) and has been shown to play a role in Treg function, survival, and expansion.10,11 We therefore assessed Treg frequencies in PBMCs and observed lower Treg frequencies in our patients (Figure 2F).
In Cd137-deficient mice, NK-cell and cytotoxic T-lymphocyte (CTL) function were diminished. Indeed, EBV-specific CTL cytotoxicity was reduced in patient CTLs compared with HDs (supplemental Figure 8A), suggesting that CD137 deficiency results in susceptibility to EBV and its related lymphomagenesis. However, CTL and NK-cell degranulation, as well as downstream TCR-signaling pathways, were intact in CD137-deficient patients (supplemental Figure 8B-E). This is reminiscent of a study investigating human FERMT3-deficient patients where there was specific cytotoxicity impairment but not general cytotoxicity.12
B-cell defects in CD137-deficient patients
CD137 is expressed in activated human B cells,13 TFH,10 and follicular dendritic cells.14 CD137 has been shown to be essential for B-cell function, including activation, affinity maturation, proliferation, and class switch recombination (CSR) through its interaction with CD137L in germinal centers.14-16 We thus hypothesized that these functions may be impaired in patient B cells. Indeed, CD137 expression was abrogated in B cells from all patients (Figure 1F), and B-cell activation was impaired (Figure 2G). Correspondingly, we mimicked T-cell–dependent and –independent stimulation on patient B cells and found defective CSR, proliferation, and lower frequencies of plasmablasts (Figure 2H-I; supplemental Figure 9). Patients’ B cells consistently showed maturation and differentiation defects with a marked reduction in memory B cells, plasmablasts, and CSR (Figure 2A,H; supplemental Figure 9A). In accordance with our data, it has been shown that CD137L signaling is required for proper activation and maturation of B cells and humoral responses.14 Alternatively, the maturation defects in B cells could also be an indirect effect of the lack of CD137 signaling in TFH cells, resulting in the reduction of these cells and, therefore, the lack of help by these specific T-cell subsets to B cells. However, because CD137 is also expressed on activated B cells (Figure 1F), although to a smaller extent than in activated T cells, we believe that the lack of CD137 on patient B cells may contribute to their proliferation and survival directly.13 Additionally, despite normal TFH cell frequency in patient 4, B-cell development was still impaired (supplemental Figure 6), supporting the notion of a primary B-cell maturation defect. Furthermore, sorted naïve B cells also showed reduced activation, proliferation, and CSR (supplemental Figure 10). Together, these findings highlight the role of CD137 in proper differentiation and function of B cells as previously reported.13
CD137 is an appealing target for immunotherapy, both for autoimmunity and malignancies. It functions as an immune suppressor, enhancing Treg expansion and ameliorating TH17 autoimmune effects.17 Conversely, CD137 is a potent immune stimulator that has been found to modulate the tumor microenvironment, enhancing T- and NK-cell cytotoxicity and their infiltration into tumors.18,19 CD137 agonistic monoclonal antibodies are currently in cancer immunotherapy trials, including combinations with checkpoint inhibitors to selectively activate tumor-targeting CTLs and NK cells aiming to provide a robust antitumor response.20,21 CD137 signaling is also used in chimeric antigen receptor T-cell immunotherapy.22 Whenever CD137 signals are enhanced, an enhanced cytotoxic response has been shown in various in vitro and in vivo models.23-25 As the binding of CD137 to CD137L triggers a bidirectional signaling,26 loss of CD137 expression in patients may also lead to lack of CD137L function. Cd137l-deficient mice develop B-cell lymphomas.15 Consistently, 2 patients developed EBV-associated B-cell lymphomas and 1 patient displayed EBV-associated lymphoproliferation, implying that CD137 deficiency is a predisposing factor for malignant transformation. The phenotypes observed in our patients show some resemblance to that of 2 other recently reported CD137-deficient patients (supplemental Table 1).27 Our findings further strengthen the role of CD137 as an appealing target for cancer immunotherapy.
Collectively, we identified novel inherited germline mutations in TNFRSF9 that allow dissection of the essential role of this costimulatory molecule in regulating human immune homeostasis. In vitro cellular phenotypes were rescued by CD28 costimulation, possibly allowing for the development of targeted therapeutics in vivo in affected patients. CD137 deficiency should be considered in patients with dysregulated immune systems presenting with autoimmunity and autoimmune lymphoproliferative syndrome–like, common variable immune deficiency, and/or EBV-related lymphoma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
For original data, please contact kaan.boztug@rud.lbg.ac.at.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and their families for participating in the study; Raúl Jimenez-Heredia (Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases [LBI-RUD]), Laurène Pfajfer (LBI-RUD), Wendy Aloo (Ludwig Maximilian University of Munich [LMU]), Carlos E. Muskus (Universidad de Antioquia [UdeA]), and Laura Naranjo (UdeA) for excellent technical assistance; Kemal Deniz (Erciyes University), Burcu S. Gorkem (Erciyes University), and Julio C. Orrego (UdeA); and James R. Lupski, Richard A. Gibbs, and Zeynep H. Coban-Akdemir for sequencing through the Baylor-Hopkins Center for Mendelian Genomics.
This work was supported by the European Research Council (ERC; Consolidator grant 820074 “iDysChart” [K.B.] and an ERC Advanced grant [C.K.]), the Jeffrey Modell Foundation (JMF; R.S.), the Care for Rare Foundation, the German Research Foundation (Gottfried-Wilhelm-Leibniz Program, CRC1054 [C.K.]), and the Else Kröner-Fresenius-Stiftung (Forschungskolleg Rare Diseases of the Immune System [C.K.]). I.S. was supported by the Care for Rare Foundation and has been a scholar of the Else Kröner-Fresenius-Stiftung. M. Thian was supported by a Cell Communication in Health and Disease (CCHD; Medical University of Vienna) doctoral fellowship and a DOC fellowship (25225) of the Austrian Academy of Sciences. A.G.D. was supported by a Deutscher Akademischer Austauschdienst/German Academic Exchange Service (DAAD) Fellowship (Thematic Program on Rare Diseases and Personalized Therapies). The Baylor-Hopkins Center for Mendelian Genomics was supported by the National Institutes of Health, National Human Genome Research Institute/National Heart, Lung, and Blood Institute grant UM1HG006542.
Authorship
Contribution: I.S. and M. Thian designed, performed, and analyzed experiments for all patients; D.M. and A.K. performed transfection of CD137 in patient cells; A.L. and A.J.S. performed and analyzed immune and genetic experiments on P2; Y.N.L. performed TCR repertoire sequencing and analysis; N.G., T. Stauber, F.G., E.U., G.S., J.M.J., E.Ö., Ö.A., T.P., M.K., A.O., C.M.T.-V., and J.L.F. provided patient samples and interpreted clinical, pathology, and/or imaging data; M.R. conducted and analyzed next-generation sequencing of P1 and P2; D.M., T.M., M.J.K., and F.H. helped supervise the study and gave intellectual input; J.D. performed variant filtering, Sanger validation, and identified the CD137 mutation in P3; R.C. conducted immunophenotyping in patients’ PBMCs; A.G.D., T. Shahin, E.A., and M. Tatematsu provided technical and experimental help; C.M.-J., I.K.C., and J.S.O. conducted and analyzed next-generation sequencing of P4; K.B., C.K., and R.S. conceptualized, initiated, and supervised the study; and I.S., M. Thian, R.S., C.K., and K.B. wrote the manuscript, which was reviewed and approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.M. is Becton Dickinson Life Sciences, Kornye, Hungary.
Correspondence: Kaan Boztug, St. Anna Children’s Cancer Research Institute (CCRI) and Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, Zimmermannplatz 10, 1090 Vienna, Austria; e-mail: kaan.boztug@ccri.at; Christoph Klein, Dr. von Hauner Children’s Hospital, University Hospital, Ludwig Maximilian University Munich, Lindwurmstr 4, 80337 Munich, Germany; e-mail: christoph.klein@med.uni-muenchen.de; and Raz Somech, Pediatric Department A and Immunology Service, Jeffrey Modell Foundation Center, Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Tel HaShomer, Israel; e-mail: raz.somech@sheba.health.gov.il.
REFERENCES
Author notes
I.S. and M. Thian contributed equally.
R.S., C.K., and K.B. contributed equally.

![Genetic and clinical presentation of 4 patients with immune dysregulation. (A) Pedigrees showing 4 families with affected individuals (patients) harboring TNFRSF9 mutations. Solid symbols indicate affected persons who were homozygous for the mutant allele; half-solid symbols, heterozygous persons; center solid symbols, unaffected persons homozygous for the mutant allele; circles, female family members; squares, male family members; double lines, consanguinity. (B) Radiographic features in patients with CD137 deficiency. Top left, Coronal T2-HASTE MR demonstrating a lobulated mass of the small intestine (white arrow) in patient 1 (P1). Top right, axial T2-HASTE STIR MR image of upper abdomen depicting hyperintense multiple metastatic liver nodules (red arrow) and bilateral renal cortical hypointense metastatic nodules (blue arrow) in patient 1. Bottom left, Chest computer tomography (CT) scan indicating right-sided hilar lymph nodes in patient 3 (P3). Bottom right, chest CT scan revealing bilateral ground-glass opacities in patient 2 (P2). (C) Top left, sections of right inguinal lymph node tissue (hematoxylin-and-eosin staining) showing numerous small regressive germinal centers (black arrow) in patient 2 (scale bar, 10 µm). Bottom right, Ki-67 immunostain in patient 2, positive in 20% to 30% of the cells exhibiting a moderate lymphocyte proliferative activity (scale bar, 10 µm). Bottom left, in situ hybridization for EBV-encoded small RNAs (EBER) displaying numerous positive cells in blue in patient 3 (scale bar, 200 µm). Top right, Multinucleated Reed-Sternberg cells (black arrows) typical of Hodgkin lymphoma (scale bar, 20 µm) in a resection of a submandibular lymph node in patient 3. (D) Schematic illustration displaying the interaction of ligands expressed on antigen-presenting cells (APCs) with their respective receptors on activated T cells, including the interaction between CD137L and CD137. (E) Localization of CD137 mutations in our patients. CD137 gene and protein domains with the 4 newly identified mutations are indicated. (F) CD137 protein expressions in activated (CD25+) CD4+ and CD8+ T cells, activated (CD86+) CD19+ B cells, and activated (interleukin 2 [IL-2] stimulated) NK cells (CD56+) in patients and healthy donors (HDs) demonstrating complete loss or markedly reduced expression in patients’ cells, measured by flow cytometry. All error bars indicate plus or minus standard error of mean (SEM). CRD, cysteine-rich domain; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; P4, patient 4; UTR, untranslated region.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/134/18/10.1182_blood.2019000644/2/m_bloodbld2019000644f1.png?Expires=1770147412&Signature=ZthnFpFwoK-LBtPAWE2kNJ0RsR7WJbuAaa4G~435ROBz~eaUo53gJqwSMkuolAAkMj56LWgfRF4WT3~PxEI~vX4cQThLMT457bTIOXE1w5e7luKVK3~sYxCpPmYCCoNIKbBmwTAVbnHs~tM8AtdBLSwxLXeeYNE9e2RcDFWql-zXEbUHjEb1sHyh08oIq3hRVNMMuMX0YbSbMFS1nuzigFb2aBrfOFvlrsHTYl7uC64Crsos7~l5f7JolWdLCU0C8TplisLthAIV1PhIJrvg-aN~GAdhddqHacAD3lf1rJ2qgm5objl4XOZyQrswd2bnO3cwBd2Dxuxd6PDD~jajmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Immunological and functional phenotypes in CD137-deficient patients. (A) T- and B-cell features in our patients: immunophenotyping revealed decreased frequencies of class-switched (CD27+ immunoglobulin D–negative [IgD−]) B cells and TFH (CD45RO+CXCR5+) cells in patient 1 (P1) compared with an HD, as measured by flow cytometry. (B) T-cell proliferation with carboxyfluorescein succinimidyl ester (CFSE) fluorescence cell incorporation assay 4 days poststimulation exhibiting reduced CD3+ T-cell proliferation in response to anti-CD3 and anti-CD3 in combination with CD137L in patients 1, 2 (P2), and 4 (P4) with partial and complete restoration upon OX40 and CD28 costimulation, respectively (****P < .0001; 2-way analysis of variance [ANOVA]). (C) Representative surface expression of CD25 on T cells as measured by flow cytometry 4 days poststimulation in patient 2 compared with an HD, demonstrating a T-cell activation defect with a compensatory effect upon CD28 costimulation. (D) Rescue of T-cell proliferation and activation via CD25 expression in patient 3 (P3) by exogenous expression of wild-type CD137. (E) Analysis of T-cell receptor γ (TRG) repertoire diversity with a tree-map representation for patients 1, 2, and 3, and age-matched healthy controls. Each colored square represents a unique clone and its size reflects its productive frequency within the repertoire. Simpson's D diversity index and Shannon's H index quantify repertoire clonality. (F) Flow cytometric expression of Tregs, displaying reduced Treg rates in patients 1 and 2 compared with an HD. (G) Top, Flow cytometric expression of CD86+ and CD25+ of CD19+CD3− cells 1 day poststimulation with CD40L in combination with IL4 showed impaired activation in patients 2 and 3. Bottom, quantification of B-cell activation, showing significantly lower B-cell activation in patient cells compared with HDs (*P < .05; ***P < .001; 2-way ANOVA). (H) Top, class-switched IgG+ and IgA+ of CD19+ cells upon various stimulations, displayed impaired class switch recombination in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, Quantification of class-switched (CD27+IgD−) CD19+ cell rates showing significantly lower frequencies in the patients (**P < .01; ***P < .001; 2-way ANOVA). (I) Top, B-cell proliferation measured by violet proliferation dye (VPD450) 4 days poststimulation showing reduced proliferating B cells in patient 3 in response to T-cell–dependent and –independent stimuli. Bottom, quantification of CD19+ proliferating cells, showing decreased rates in patients (*P <.05; 2-way ANOVA). All error bars indicate plus or minus SEM. CDR, complementarity-determining region; CpG, cytosine guanine dinucleotide; IGH, immunoglobulin heavy; Unstim, unstimulated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/134/18/10.1182_blood.2019000644/2/m_bloodbld2019000644f2.png?Expires=1770147412&Signature=D8WgfN9ydPmbRXjfnq7h76XFgHykYTdL-57-r89Yv6o3SL5jzd9U8CL6hYr8WsSzpr5c7FlPi2CGcGeY3IZHGIE2s0l8Bvl27cfdZBzwpfQ6fW~QrsTe7l8fYzd~moeiJm9DYRERSbO1NQ34~~HFyajB~iDhIrPHRtghx~WG4eye6OHDxSWut2klWkzTiU82TbGYafuobREm3Dm1cicoLrWcegNWagTlptcCsF-5KpCvNqPLwXtDEaRMwvAUbyGxm9xcOqJlP~sM4CLgVaFkscOptmjokuGBTpFe2EPnqJu79b3CDs6VgD~Gvpc~yNuB2Ic7uLsODsJnifC1kfnFWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal