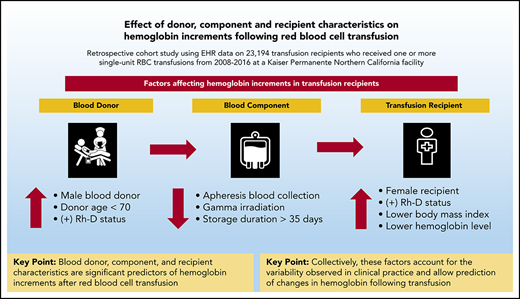
Key Points
Blood donor, component, and recipient characteristics are significant predictors of hemoglobin increments after RBC transfusion.
Collectively, these factors account for the variation observed in practice and allow prediction of changes in hemoglobin with transfusion.
Abstract
Significant research has focused individually on blood donors, product preparation and storage, and optimal transfusion practice. To better understand the interplay between these factors on measures of red blood cell (RBC) transfusion efficacy, we conducted a linked analysis of blood donor and component data with patients who received single-unit RBC transfusions between 2008 and 2016. Hemoglobin levels before and after RBC transfusions and at 24- and 48-hour intervals after transfusion were analyzed. Generalized estimating equation linear regression models were fit to examine hemoglobin increments after RBC transfusion adjusting for donor and recipient demographic characteristics, collection method, additive solution, gamma irradiation, and storage duration. We linked data on 23 194 transfusion recipients who received one or more single-unit RBC transfusions (n = 38 019 units) to donor demographic and component characteristics. Donor and recipient sex, Rh-D status, collection method, gamma irradiation, recipient age and body mass index, and pretransfusion hemoglobin levels were significant predictors of hemoglobin increments in univariate and multivariable analyses (P < .01). For hemoglobin increments 24 hours after transfusion, the coefficient of determination for the generalized estimating equation models was 0.25, with an estimated correlation between actual and predicted values of 0.5. Collectively, blood donor demographic characteristics, collection and processing methods, and recipient characteristics accounted for significant variation in hemoglobin increments related to RBC transfusion. Multivariable modeling allows the prediction of changes in hemoglobin using donor-, component-, and patient-level characteristics. Accounting for these factors will be critical for future analyses of donor and component factors, including genetic polymorphisms, on posttransfusion increments and other patient outcomes.
Introduction
The role of blood donor demographic and genetic characteristics, component processing, and recipient factors on transfusion efficacy is an area of high interest in transfusion medicine research.1,2 Although inherent variability in the blood donor population is known to affect blood component quality, the impact on transfusion recipient outcomes remains unclear.3,,-6 In addition, differences in blood collection methods (eg, manual or apheresis), anticoagulants and storage solutions, and leukoreduction result in products with different physiological and biochemical characteristics through storage.7,8
The development of linked blood donor–component–recipient databases provides the capacity to interrogate relevant questions in transfusion medicine.9,-11 Donor and component characteristics may affect rates of in vitro hemolysis or the hemoglobin content of a unit of RBC. RBC products from male donors are known to have higher total hemoglobin content than those from their female counterparts; however, rates of storage hemolysis are higher in male donor–derived RBC units and vary with donor age in both sexes.12,13 Differences in hemolysis may be clinically relevant given the reported (albeit controversial) associations of blood donor age and sex with adverse outcomes in transfusion recipients.3,5,6
Blood collection method, component manufacturing methods, and choice of additive solutions have also been shown to affect characteristics of transfused products and may influence product quality.14,15 Both gamma irradiation and prolonged storage duration of RBC components have been associated with increased in vitro and in vivo hemolysis.16,,-19 In addition, recipient characteristics are relevant to outcomes of RBC transfusion. Circulating blood volumes differ with recipient sex and body mass index (BMI) and play a role in adverse transfusion reactions.20,-22 A transfusion recipient’s inflammatory state is also known to be critical in the pathogenesis of transfusion-related acute lung injury and sickle cell disease, and recipient comorbidities have been associated with RBC alloimmunization.23,24
The advent of precision medicine seeks to tailor health care delivery to achieve maximum benefit based on an understanding of the factors that contribute to health and disease at individual and population levels.25 One measure of transfusion efficacy is the hemoglobin increment after transfusion of each packed RBC unit. The common and historical assumption among clinicians is that transfusion of a single RBC unit results in a 1-g/dL increase in hemoglobin levels.26 However, there are few data examining the relative contribution of donor, component collection and manufacturing, and recipient characteristics on outcomes after transfusion. We sought to quantitatively evaluate the role of these factors on hemoglobin increments related to RBC transfusion and hypothesized that collectively they would account for the significant variation seen in clinical practice.
Materials and methods
Study design and setting
This retrospective cohort study was conducted by using electronic health record data from Kaiser Permanente Northern California (KPNC), which serves a population of >4 million patients. All KPNC hospitals and clinics use a common electronic health record (Epic, Verona, WI). The KPNC and University of California, San Francisco, Institutional Review Boards approved this study.
Blood donor and component characteristics
Information on blood donors, donations, and processing was obtained from databases of the Vitalant Research Institute, which collected blood at multiple blood centers and supplied blood components to 9 of 21 KPNC facilities. All blood products were leukocyte-depleted by prestorage filtration, and all donors were at least 16 years old. Data collected included donor demographic characteristics (age and sex), RBC collection date and method (whole blood or apheresis), and blood component characteristics (ABO/Rh-status, irradiation status, and additive solution) for each unit collected. In a subset of donations from 1 blood center, details regarding donor smoking status and timing of gamma irradiation were included.
Recipient cohort and characteristics
The study included all adult KPNC inpatients and outpatients who received a single RBC unit during one or more transfusion episodes at 9 medical centers from January 1, 2008, to June 30, 2016. Recipient details included age, sex, and BMI, as well as the storage duration, transfusion date and time, and product identification numbers of transfused RBCs. We collected hemoglobin levels measured by the clinical laboratory before and after each RBC transfusion event. To assess the role of patient diagnoses, we grouped diagnosis codes from the ninth and 10th revisions of the International Classification of Diseases by using Health Care Utilization Project (www.ahrq.gov/data/hcup) Clinical Classifications Software categories.
Outcomes
The primary outcome was the recipient’s hemoglobin increment after a single RBC unit transfusion. Changes in hemoglobin levels were calculated as the difference in the proximate pretransfusion hemoglobin levels and posttransfusion hemoglobin levels at time intervals after transfusion. Transfusions were excluded from analyses if there were no pretransfusion or posttransfusion hemoglobin levels in the specific time periods or if the patient received multiple RBC transfusions during a single transfusion episode.
Pretransfusion hemoglobin levels were defined as the proximate hemoglobin level before transfusion; for this analysis, the time from determination of the pretransfusion hemoglobin level to the blood transfusion was limited to ≤18 hours. Initial hemoglobin levels within 18 hours after transfusion (Hbpost) and for 24 hours (Hbpost24) and 48 hours (Hbpost48) after the transfusion periods were assessed. Hbpost24 was defined as the posttransfusion hemoglobin level closest to 24 hours after transfusion, within an 18- to 36-hour posttransfusion window, with no intervening transfusion. Hbpost48 was the posttransfusion hemoglobin level closest to 48 hours after transfusion, within a 36- to 60-hour window, with no intervening transfusion.
Statistical Analysis
Donor, component, and recipient characteristics were linked by using product identification numbers of recipients’ transfused RBCs. We then quantitatively evaluated associations between these factors and hemoglobin increments for each transfused RBC unit. We first examined univariate associations between hemoglobin increments and donor sex, age, and Rh status, as well as recipient characteristics, including age, sex, BMI, and pretransfusion hemoglobin levels. Given conflicting findings regarding bleeding risk related to blood type, we analyzed changes in hemoglobin for blood group O vs non-O.27 We also examined hemoglobin increments based on blood collection method (whole blood or apheresis-derived), gamma irradiation, and RBC storage duration. Given the potential for selection bias due to infrequently used storage solutions, we focused analyses on additive solutions 1 and 3 (AS-1 and AS-3, respectively). To account for the possibility of indication bias with receipt of gamma irradiated RBCs, hemoglobin increments were additionally evaluated in patients whose primary diagnoses was related to neoplasm (eg, leukemia, lymphoma, solid tumor, receipt of chemotherapy).
We then examined multivariable associations between all exposures and hemoglobin increments simultaneously. Generalized estimating equations (GEE) were used to account for correlated transfusion events in a given recipient. Models were fit to examine hemoglobin increments after RBC transfusion, adjusting for the aforementioned donor, component, and recipient characteristics. Interactions between individual factors were examined to detect the presence of effect modification. Intervals from the time of laboratory testing (pretransfusion and posttransfusion hemoglobin levels) in relation to transfusion time, as well as the year of transfusion, were accounted for. Separate models were fit to examine hemoglobin increments initially after transfusion and for 24- and 48-hour periods after transfusion. We selected the correlation structure with the best model fit based on the quasi-likelihood under the independence model criterion (QIC). As secondary analyses, we fit the aforementioned models for the transfusion episodes available in all 3 posttransfusion time periods (initial, 24-hour, and 48-hour) as well as for inpatient vs outpatient transfusion episodes.
Data are presented as counts and percentages, means with standard deviations (SDs), or medians and interquartile ranges (IQRs). Accordingly, χ2 tests for equal proportion, Student t tests, or Wilcoxon rank sum tests were used to test differences. Regression results were reported as coefficients with 95% confidence intervals representing the mean hemoglobin increment after RBC transfusion for that variable. To assess model fit, we calculated the coefficient of determination (R2) for GEE models and assessed calibration by using plots of predicted vs actual hemoglobin increments and corresponding correlation.28 Two-sided P values <.05 were considered to be statistically significant. Statistical analyses were performed by using Stata version 14.1 (StataCorp, College Station, TX), R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria), and SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
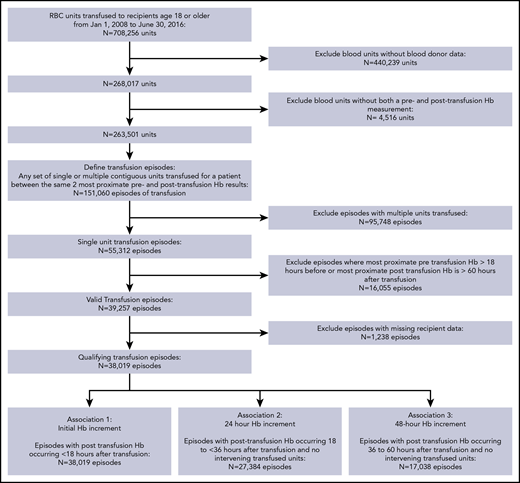
We identified 139 433 patients who received 708 256 RBC transfusions at KPNC facilities from 2008 to 2016 (Figure 1). Blood donor demographic data were available for 9 of 21 medical centers and were linked to 63 162 recipients who received 268 017 RBC transfusions. Among the linked donor–recipient transfusions, hemoglobin data before and after transfusion were available for 151 060 transfusion episodes, and 55 312 of these 151 060 transfusion episodes represented a single RBC unit transfusion. Single-unit transfusion episodes were further limited to those with hemoglobin determinations within 18 hours before transfusion and 60 hours after transfusion, resulting in 38 019 informative transfusion episodes occurring in inpatient (n = 29 045) and outpatient (n = 8974) settings. Hemoglobin levels within the initial posttransfusion, 24-hour, and 48-hour periods occurred without additional interval transfusions in 38 019, 27 384, and 17 038 episodes, respectively.
For the 38 019 informative transfusion episodes, the median blood donor age was 47 years (IQR, 29-58 years), and 57.3% of transfused RBC units were from male donors (Table 1). Median transfusion recipient age was 72 years (IQR, 61-81 years), and 49.9% were male. Overall, 21.4% of all transfused RBC units were derived from apheresis collections, 70.6% were stored in AS-3, 6.5% underwent gamma irradiation, and the median storage duration was 26 days (IQR, 20-32 days). These characteristics were similar for recipients for whom 24- and 48-hour hemoglobin levels were available (supplemental Table 1, available on the Blood Web site). Recipient demographic characteristics were also similar for episodes in which donor data were not available and for recipients of multiple unit RBC transfusion episodes not included in the analysis (supplemental Table 2).
Donor, component, and recipient characteristics
| No. of informative transfusion episodes | 38 019 | ||
| Blood donor characteristics | |||
| Male sex, % | 57.3 | ||
| Age, median (IQR), y | 47 (29-58) | ||
| (+) Rh status, % | 81.7 | ||
| ABO status, % | |||
| A | 36.9 | ||
| B | 1.9 | ||
| AB | 12.3 | ||
| O | 48.9 | ||
| Blood component characteristics | |||
| Gamma irradiated, % | 6.5 | ||
| RBC storage duration, median (IQR), d | 26 (20-32) | ||
| Apheresis-derived, % | 21.4 | ||
| Storage solution, % | |||
| AS-1 | 23.9 | ||
| AS-3 | 70.6 | ||
| CPDA | 4.2 | ||
| AS-5 | 1.3 | ||
| Transfusion recipient characteristics | |||
| Male sex, % | 49.9 | ||
| Age, median (IQR), y | 72 (61-81) | ||
| BMI, median (IQR), kg/m2 | 26.3 (23.0-31.0) | ||
| (+) Rh status, % | 87.9 | ||
| ABO status, % | |||
| A | 36.8 | ||
| B | 3.8 | ||
| AB | 13.7 | ||
| O | 45.7 | ||
| Pretransfusion Hb, mean SD, g/dL | 8.00 (0.88) | ||
| Posttransfusion Hb, mean SD, g/dL | 9.04 (1.15) | ||
| Time between pretransfusion Hb and transfusion, median (IQR), h | 5.0 (2.6-8.5) | ||
| Time between transfusion and posttransfusion Hb, median (IQR), h | 5.8 (3.7-11.4) | ||
| No. of informative transfusion episodes | 38 019 | ||
| Blood donor characteristics | |||
| Male sex, % | 57.3 | ||
| Age, median (IQR), y | 47 (29-58) | ||
| (+) Rh status, % | 81.7 | ||
| ABO status, % | |||
| A | 36.9 | ||
| B | 1.9 | ||
| AB | 12.3 | ||
| O | 48.9 | ||
| Blood component characteristics | |||
| Gamma irradiated, % | 6.5 | ||
| RBC storage duration, median (IQR), d | 26 (20-32) | ||
| Apheresis-derived, % | 21.4 | ||
| Storage solution, % | |||
| AS-1 | 23.9 | ||
| AS-3 | 70.6 | ||
| CPDA | 4.2 | ||
| AS-5 | 1.3 | ||
| Transfusion recipient characteristics | |||
| Male sex, % | 49.9 | ||
| Age, median (IQR), y | 72 (61-81) | ||
| BMI, median (IQR), kg/m2 | 26.3 (23.0-31.0) | ||
| (+) Rh status, % | 87.9 | ||
| ABO status, % | |||
| A | 36.8 | ||
| B | 3.8 | ||
| AB | 13.7 | ||
| O | 45.7 | ||
| Pretransfusion Hb, mean SD, g/dL | 8.00 (0.88) | ||
| Posttransfusion Hb, mean SD, g/dL | 9.04 (1.15) | ||
| Time between pretransfusion Hb and transfusion, median (IQR), h | 5.0 (2.6-8.5) | ||
| Time between transfusion and posttransfusion Hb, median (IQR), h | 5.8 (3.7-11.4) | ||
CPDA, citrate-phosphate-dextrose-adenine.
The mean ± SD pretransfusion hemoglobin level was 8.00 ± 0.88 g/dL and was measured 5 hours (IQR, 2.6-8.5 hours) before transfusion. The initial posttransfusion hemoglobin level was measured 5.8 hours (IQR, 3.7-11.4 hours) after transfusion, and the median times from transfusion to Hbpost24 and Hbpost48 were 23.7 hours (IQR, 20.4-28.9 hours) and 43.7 hours (IQR, 40.0-50.5 hours), respectively.
For the initial posttransfusion period (Table 2), the hemoglobin increment was 1.04 ± 0.89 g/dL. Larger hemoglobin increments were seen in recipients of RBC units from male donors (mean ± SD, 1.08 ± 0.87 g/dL) compared with female donors (1.01 ± 0.89 g/dL; P < .001). Hemoglobin increments were also larger in recipients of whole blood–derived RBC units (1.07 ± 0.88 g/dL) compared with apheresis-derived RBC units (0.97 ± 0.87 g/dL; P < .001). Similar findings were observed for Hbpost24 and Hbpost48 posttransfusion periods (supplemental Table 3). Hemoglobin increments were reduced for transfusion of RBC units from donors aged >70 years compared with all other donors at 24- and 48-hour posttransfusion periods (P = .017 and .015, respectively) (supplemental Table 4). Positive donor or recipient Rh-D status was associated with larger hemoglobin increments compared with negative Rh-D status (P < .001).
Univariate changes in Hb levels after transfusion (N = 38 019)
| Variable . | Hb increment, g/dL . | P* . |
|---|---|---|
| Overall change in Hb level | 1.04 ± 0.89 | |
| Blood donor characteristic | ||
| Donor sex | <.001 | |
| Male | 1.08 ± 0.87 | |
| Female | 1.01 ± 0.89 | |
| Donor age | .43 | |
| <20 y | 1.05 ± 0.89 | |
| 20-44 y | 1.04 ± 0.89 | |
| 45-70 y | 1.05 ± 0.88 | |
| >70 y | 1.02 ± 0.84 | |
| Donor Rh status | <.001 | |
| Negative | 1.01 ± 0.89 | |
| Positive | 1.06 ± 0.87 | |
| Donor ABO blood group† | .14 | |
| O | 1.04 ± 0.90 | |
| Non-O | 1.06 ± 0.89 | |
| Blood component characteristic | ||
| Collection method | <.001 | |
| Apheresis-derived | 0.97 ± 0.87 | |
| Whole blood | 1.07 ± 0.88 | |
| Storage duration‡ | .09 | |
| 1-21 d | 1.04 ± 0.89 | |
| 22-28 d | 1.05 ± 0.90 | |
| 29-35 d | 1.06 ± 0.86 | |
| 36-42 d | 1.05 ± 0.84 | |
| RBC solution, % | .80 | |
| AS-1 | 1.04 ± 0.89 | |
| AS-3 | 1.05 ± 0.87 | |
| Gamma irradiation | <.001 | |
| Yes | 0.98 ± 0.81 | |
| No | 1.06 ± 0.88 | |
| Transfusion recipient characteristic | ||
| Recipient sex | <.0001 | |
| Male | 0.91 ± 0.83 | |
| Female | 1.19 ± 0.91 | |
| Recipient age | <.0001 | |
| 18-55 y | 0.96 ± 0.92 | |
| 56-69 y | 0.98 ± 0.87 | |
| 70-80 y | 1.05 ± 0.86 | |
| >80 y | 1.16 ± 0.87 | |
| Recipient BMI | <.0001 | |
| Underweight | 1.44 ± 0.96 | |
| Normal | 1.16 ± 0.92 | |
| Overweight | 1.03 ± 0.86 | |
| Obese | 0.89 ± 0.80 | |
| Recipient Rh status | <.0001 | |
| Negative | 1.00 ± 0.88 | |
| Positive | 1.06 ± 0.88 | |
| Recipient ABO blood group† | ||
| O | 1.04 ± 0.89 | |
| Non-O | 1.05 ± 0.86 | |
| Pretransfusion Hb level | <.0001 | |
| <7 g/dL | 1.26 ± 0.95 | |
| 7-8 g/dL | 1.11 ± 0.82 | |
| >8 g/dL | 0.96 ± 0.89 |
| Variable . | Hb increment, g/dL . | P* . |
|---|---|---|
| Overall change in Hb level | 1.04 ± 0.89 | |
| Blood donor characteristic | ||
| Donor sex | <.001 | |
| Male | 1.08 ± 0.87 | |
| Female | 1.01 ± 0.89 | |
| Donor age | .43 | |
| <20 y | 1.05 ± 0.89 | |
| 20-44 y | 1.04 ± 0.89 | |
| 45-70 y | 1.05 ± 0.88 | |
| >70 y | 1.02 ± 0.84 | |
| Donor Rh status | <.001 | |
| Negative | 1.01 ± 0.89 | |
| Positive | 1.06 ± 0.87 | |
| Donor ABO blood group† | .14 | |
| O | 1.04 ± 0.90 | |
| Non-O | 1.06 ± 0.89 | |
| Blood component characteristic | ||
| Collection method | <.001 | |
| Apheresis-derived | 0.97 ± 0.87 | |
| Whole blood | 1.07 ± 0.88 | |
| Storage duration‡ | .09 | |
| 1-21 d | 1.04 ± 0.89 | |
| 22-28 d | 1.05 ± 0.90 | |
| 29-35 d | 1.06 ± 0.86 | |
| 36-42 d | 1.05 ± 0.84 | |
| RBC solution, % | .80 | |
| AS-1 | 1.04 ± 0.89 | |
| AS-3 | 1.05 ± 0.87 | |
| Gamma irradiation | <.001 | |
| Yes | 0.98 ± 0.81 | |
| No | 1.06 ± 0.88 | |
| Transfusion recipient characteristic | ||
| Recipient sex | <.0001 | |
| Male | 0.91 ± 0.83 | |
| Female | 1.19 ± 0.91 | |
| Recipient age | <.0001 | |
| 18-55 y | 0.96 ± 0.92 | |
| 56-69 y | 0.98 ± 0.87 | |
| 70-80 y | 1.05 ± 0.86 | |
| >80 y | 1.16 ± 0.87 | |
| Recipient BMI | <.0001 | |
| Underweight | 1.44 ± 0.96 | |
| Normal | 1.16 ± 0.92 | |
| Overweight | 1.03 ± 0.86 | |
| Obese | 0.89 ± 0.80 | |
| Recipient Rh status | <.0001 | |
| Negative | 1.00 ± 0.88 | |
| Positive | 1.06 ± 0.88 | |
| Recipient ABO blood group† | ||
| O | 1.04 ± 0.89 | |
| Non-O | 1.05 ± 0.86 | |
| Pretransfusion Hb level | <.0001 | |
| <7 g/dL | 1.26 ± 0.95 | |
| 7-8 g/dL | 1.11 ± 0.82 | |
| >8 g/dL | 0.96 ± 0.89 |
P values correspond to Student t tests.
Non-O blood group includes A, B, and AB blood types.
Increments for other storage solutions included in supplemental Table 3.
There were no significant differences in hemoglobin increments related to RBC storage duration in univariable comparisons (Table 2; supplemental Table 3). Recipients of irradiated RBC units vs unirradiated RBC units had smaller hemoglobin increments (0.98 vs 1.06; P < .001), and these differences did not vary according to RBC storage duration or time from gamma irradiation (supplemental Tables 5 and 6). Hemoglobin increments were reduced for recipients of irradiated units with and without oncologic diagnoses (Table 3).
Hb increments in relation to oncologic Dx and gamma irradiation status
| Variable . | Initial Hb increment, g/dL* . | 24-h Hb increment, g/dL* . | ||
|---|---|---|---|---|
| Nononcologic Dx (n = 34 516) . | Oncologic Dx (n = 3503) . | Nononcologic Dx (n = 24 697) . | Oncologic Dx (n = 2687) . | |
| Unirradiated | ||||
| Pre-Tx Hb | 8.03 (0.87) | 7.96 (0.89) | 8.05 (0.82) | 7.96 (0.80) |
| Post-Tx Hb | 9.08 (1.14) | 9.01 (1.24) | 9.09 (1.18) | 8.99 (1.14) |
| Hb increment | 1.06 (0.88) | 1.06 (0.96) | 1.04 (0.90) | 1.03 (0.92) |
| Irradiated | ||||
| Pre-Tx Hb | 7.78 (0.84) | 7.64 (0.73) | 7.77 (0.84) | 7.62 (0.73) |
| Post-Tx Hb | 8.74 (1.13) | 8.53 (1.09) | 8.75 (1.16) | 8.44 (0.99) |
| Hb increment | 1.00 (0.81) | 0.92 (0.87) | 0.98 (0.90) | 0.82 (0.82) |
| Variable . | Initial Hb increment, g/dL* . | 24-h Hb increment, g/dL* . | ||
|---|---|---|---|---|
| Nononcologic Dx (n = 34 516) . | Oncologic Dx (n = 3503) . | Nononcologic Dx (n = 24 697) . | Oncologic Dx (n = 2687) . | |
| Unirradiated | ||||
| Pre-Tx Hb | 8.03 (0.87) | 7.96 (0.89) | 8.05 (0.82) | 7.96 (0.80) |
| Post-Tx Hb | 9.08 (1.14) | 9.01 (1.24) | 9.09 (1.18) | 8.99 (1.14) |
| Hb increment | 1.06 (0.88) | 1.06 (0.96) | 1.04 (0.90) | 1.03 (0.92) |
| Irradiated | ||||
| Pre-Tx Hb | 7.78 (0.84) | 7.64 (0.73) | 7.77 (0.84) | 7.62 (0.73) |
| Post-Tx Hb | 8.74 (1.13) | 8.53 (1.09) | 8.75 (1.16) | 8.44 (0.99) |
| Hb increment | 1.00 (0.81) | 0.92 (0.87) | 0.98 (0.90) | 0.82 (0.82) |
All presented data are mean ± SD.
Dx, diagnosis; Tx, transfusion.
Pretransfusion Hb levels within 18 hours of Tx, and Hb increments within 18-hour period after Tx for the initial period and between the 18- and 36-hour period after Tx for 24-hour increment. Tx recipients of gamma irradiated RBCs compared with unirradiated RBCs had smaller Hb increments in patients with and without an oncologic Dx. All P values <.001 by Student t tests.
Female transfusion recipients and patients with lower BMI had larger hemoglobin increments compared with male and overweight or obese individuals (Table 2). Hemoglobin increments were inversely proportional to hemoglobin levels, with larger increments associated with progressive degrees of pretransfusion anemia. The effects of individual donor, component, and recipient characteristics seemed to be additive, with the lowest increments observed in male recipients of irradiated or apheresis-derived units from female donors (Tables 4 and 5). Lastly, hemoglobin increments did not vary significantly according to donor smoking status (supplemental Table 7).
Hb increments for donor and recipient sex and gamma irradiation status*
| Variable . | Male blood donor . | Female blood donor . | ||
|---|---|---|---|---|
| Unirradiated (n = 20 275) . | Irradiated (n = 1505) . | Unirradiated (n = 15 267) . | Irradiated (n = 972) . | |
| Female recipient | ||||
| Pre-Tx Hb | 8.02 (0.88) | 7.80 (0.86) | 8.00 (0.90) | 7.79 (0.84) |
| Post-Tx Hb | 9.25 (1.18) | 8.99 (1.20) | 9.14 (1.17) | 8.88 (1.18) |
| Hb increment | 1.23 (0.93) | 1.18 (0.96) | 1.14 (0.89) | 1.08 (0.84) |
| Male recipient | ||||
| Pre-Tx Hb | 8.03 (0.86) | 7.76 (0.83) | 8.04 (0.88) | 7.75 (0.85) |
| Post-Tx Hb | 8.97 (1.11) | 8.64 (1.08) | 8.92 (1.13) | 8.50 (1.10) |
| Hb increment | 0.93 (0.83) | 0.88 (0.79) | 0.88 (0.84) | 0.74 (0.77) |
| Variable . | Male blood donor . | Female blood donor . | ||
|---|---|---|---|---|
| Unirradiated (n = 20 275) . | Irradiated (n = 1505) . | Unirradiated (n = 15 267) . | Irradiated (n = 972) . | |
| Female recipient | ||||
| Pre-Tx Hb | 8.02 (0.88) | 7.80 (0.86) | 8.00 (0.90) | 7.79 (0.84) |
| Post-Tx Hb | 9.25 (1.18) | 8.99 (1.20) | 9.14 (1.17) | 8.88 (1.18) |
| Hb increment | 1.23 (0.93) | 1.18 (0.96) | 1.14 (0.89) | 1.08 (0.84) |
| Male recipient | ||||
| Pre-Tx Hb | 8.03 (0.86) | 7.76 (0.83) | 8.04 (0.88) | 7.75 (0.85) |
| Post-Tx Hb | 8.97 (1.11) | 8.64 (1.08) | 8.92 (1.13) | 8.50 (1.10) |
| Hb increment | 0.93 (0.83) | 0.88 (0.79) | 0.88 (0.84) | 0.74 (0.77) |
All presented data are mean ± SD. Pretransfusion Hb level within 18 hours of transfusion (Tx) and Hb increments within 18-hour period after Tx. Hb values are given in grams per deciliter.
P < .001 for mean trend in Hb increments across donor, component, and recipient status.
Hb increments for donor and recipient sex and blood collection method*
| Variable . | Whole blood collection . | Apheresis collection . | ||
|---|---|---|---|---|
| Male donor (n = 14 875) . | Female donor (n = 15 025) . | Male donor (n = 6905) . | Female donor (n = 1214) . | |
| Female recipient | ||||
| Pre-Tx Hb | 7.97 (0.88) | 7.98 (0.89) | 8.04 (0.88) | 7.92 (0.88) |
| Post-Tx Hb | 9.25 (1.18) | 9.13 (1.16) | 9.15 (1.15) | 8.96 (1.12) |
| Hb increment | 1.29 (0.93) | 1.14 (0.89) | 1.10 (0.90) | 1.04 (0.82) |
| Male recipient | ||||
| Pre-Tx Hb | 7.98 (0.86) | 8.00 (0.88) | 8.05 (0.86) | 8.09 (0.88) |
| Post-Tx Hb | 8.96 (1.11) | 8.88 (1.13) | 8.90 (1.10) | 8.88 (1.18) |
| Hb increment | 0.97 (0.82) | 0.88 (0.83) | 0.85 (0.82) | 0.79 (0.86) |
| Variable . | Whole blood collection . | Apheresis collection . | ||
|---|---|---|---|---|
| Male donor (n = 14 875) . | Female donor (n = 15 025) . | Male donor (n = 6905) . | Female donor (n = 1214) . | |
| Female recipient | ||||
| Pre-Tx Hb | 7.97 (0.88) | 7.98 (0.89) | 8.04 (0.88) | 7.92 (0.88) |
| Post-Tx Hb | 9.25 (1.18) | 9.13 (1.16) | 9.15 (1.15) | 8.96 (1.12) |
| Hb increment | 1.29 (0.93) | 1.14 (0.89) | 1.10 (0.90) | 1.04 (0.82) |
| Male recipient | ||||
| Pre-Tx Hb | 7.98 (0.86) | 8.00 (0.88) | 8.05 (0.86) | 8.09 (0.88) |
| Post-Tx Hb | 8.96 (1.11) | 8.88 (1.13) | 8.90 (1.10) | 8.88 (1.18) |
| Hb increment | 0.97 (0.82) | 0.88 (0.83) | 0.85 (0.82) | 0.79 (0.86) |
All presented data are mean ± SD. Pretransfusion Hb level within 18 hours of transfusion (Tx) and Hb increments within 18-hour period after Tx. Hb values are given in grams per deciliter.
P < .001 for mean trend in Hb increments across donor, component, and recipient status.
Table 6 shows regression estimates from the multivariate model for the initial posttransfusion period, accounting for correlated transfusion events. Regression coefficients estimate the mean hemoglobin increment after transfusion for each level change (for categorical variables) or unit increase (for continuous variables) of each predictor. The aforementioned univariate findings of donor, component, and recipient characteristics remained significantly associated with changes in hemoglobin levels for the initial posttransfusion period as well as within the 24- and 48-hour windows (supplemental Table 8). In addition, hemoglobin increments were smaller for components stored in AS-3 vs AS-1 (–0.05 g/dL; P = .01). Donor age and RBC storage duration were not significant predictors initially after transfusion, but donor age >70 years and storage duration >35 days exhibited small but statistically significant associations with decreased hemoglobin increments at 24- and 48-hour periods after transfusion (all, P < .05).
Regression estimates for hemoglobin increment in the initial posttransfusion period (n = 38 019)
| Characteristic . | Change in hemoglobin (95% CI) . | P . |
|---|---|---|
| Male donor | 0.10 (0.08 to 0.12) | <.01 |
| Donor age (reference, <20 y) | ||
| 20-45 | 0.00 (–0.03 to 0.03) | .95 |
| 45-70 | 0.00 (–0.02 to 0.03) | .81 |
| >70 | −0.03 (–0.08 to 0.01) | .14 |
| Donor Rh-positive status | 0.00 (–0.04 to 0.03) | .86 |
| Donor ABO status (ref: non-O) | ||
| Blood type O | −0.01 (–0.07 to 0.04) | .65 |
| Whole blood collection | 0.16 (0.13 to 0.18) | <.001 |
| Storage duration (ref: 1-21 d) | ||
| 22-28 | −0.00 (–0.03 to 0.03) | .41 |
| 29-35 | −0.00 (–0.02 to 0.01) | .73 |
| 36-42 | −0.02 (–0.04 to 0.01) | .27 |
| RBC additive solution (reference, AS-1)* | ||
| AS-3 | −0.07 (–0.12 to −0.02) | .01 |
| Gamma irradiation | −0.06 (–0.10 to −0.02) | .002 |
| Female recipient | 0.28 (0.26 to 0.29) | <.001 |
| Recipient age† | 0.04 (0.04 to 0.05) | <.001 |
| Recipient BMI | −0.02 (–0.02 to −0.02) | <.001 |
| Recipient Rh-positive status | 0.06 (0.01 to 0.10) | .01 |
| Recipient ABO status (reference, non-O) | ||
| Blood type O | 0.01 (–0.06 to 0.08) | .87 |
| Pre-transfusion Hb level | −0.18 (–0.20 to −0.17) | <.001 |
| Hours between pre-Tx measurement and Tx | 0.01 (0.01 to 0.01) | <.001 |
| Hours between Tx and post-Tx measurement | 0.01 (0.01 to 0.01) | <.001 |
| Tx year | −0.02 (–0.02 to −0.01) | <.001 |
| Characteristic . | Change in hemoglobin (95% CI) . | P . |
|---|---|---|
| Male donor | 0.10 (0.08 to 0.12) | <.01 |
| Donor age (reference, <20 y) | ||
| 20-45 | 0.00 (–0.03 to 0.03) | .95 |
| 45-70 | 0.00 (–0.02 to 0.03) | .81 |
| >70 | −0.03 (–0.08 to 0.01) | .14 |
| Donor Rh-positive status | 0.00 (–0.04 to 0.03) | .86 |
| Donor ABO status (ref: non-O) | ||
| Blood type O | −0.01 (–0.07 to 0.04) | .65 |
| Whole blood collection | 0.16 (0.13 to 0.18) | <.001 |
| Storage duration (ref: 1-21 d) | ||
| 22-28 | −0.00 (–0.03 to 0.03) | .41 |
| 29-35 | −0.00 (–0.02 to 0.01) | .73 |
| 36-42 | −0.02 (–0.04 to 0.01) | .27 |
| RBC additive solution (reference, AS-1)* | ||
| AS-3 | −0.07 (–0.12 to −0.02) | .01 |
| Gamma irradiation | −0.06 (–0.10 to −0.02) | .002 |
| Female recipient | 0.28 (0.26 to 0.29) | <.001 |
| Recipient age† | 0.04 (0.04 to 0.05) | <.001 |
| Recipient BMI | −0.02 (–0.02 to −0.02) | <.001 |
| Recipient Rh-positive status | 0.06 (0.01 to 0.10) | .01 |
| Recipient ABO status (reference, non-O) | ||
| Blood type O | 0.01 (–0.06 to 0.08) | .87 |
| Pre-transfusion Hb level | −0.18 (–0.20 to −0.17) | <.001 |
| Hours between pre-Tx measurement and Tx | 0.01 (0.01 to 0.01) | <.001 |
| Hours between Tx and post-Tx measurement | 0.01 (0.01 to 0.01) | <.001 |
| Tx year | −0.02 (–0.02 to −0.01) | <.001 |
Pretransfusion Hb level within 18 hours of transfusion (Tx) and change in Hb within 18-hour period after Tx. Hb changes are given in grams per deciliter. CI, confidence interval.
Regression estimates for other storage solutions included in appendix tables.
Coefficient for recipient age corresponds to change in mean outcome associated with 10-year increase.
The estimated coefficients of determination (R2) for the GEE multivariate models were 0.18, 0.25, and 0.28 for the initial, 24-hour, and 48-hour posttransfusion increments, respectively.28 Estimated correlations between the predicted and actual hemoglobin increments were 0.43, 0.50, and 0.53 for the initial, 24-hour, and 48-hour increments. In the secondary analyses restricted to the 17 038 episodes available for all 3 posttransfusion time periods and between inpatient and outpatient transfusion episodes, results were comparable to the main analyses (supplemental Tables 9 and 10). Regression coefficients from a simplified GEE model were used to estimate the initial posttransfusion hemoglobin increment representing the spectrum of combinations of donor, component, and recipient characteristics for a pretransfusion hemoglobin level of 7 g/dL (Table 7).
Predicted Hb increment after RBC transfusion for individual donor, component, and recipient factors for Hb level of 7 g/dL
| Female donor (0) | Male donor (+0.1) |
| Donor/recipient Rh-D negative (–0.06) | Donor/recipient Rh-D positive (0) |
| Apheresis collection (0) | Whole blood collection (+0.16) |
| Irradiated unit (–0.08) | Unirradiated unit (0) |
| AS-3 (–0.06) | AS-1 (0) |
| 60-year-old male recipient (0) | 85-year-old female recipient (+0.4) |
| BMI – 30 (–0.5) | BMI – 18 (-0.3) |
| Cumulative Hb increment: 0.59 g/dL | Cumulative Hb increment: 1.65 g/dL |
| Female donor (0) | Male donor (+0.1) |
| Donor/recipient Rh-D negative (–0.06) | Donor/recipient Rh-D positive (0) |
| Apheresis collection (0) | Whole blood collection (+0.16) |
| Irradiated unit (–0.08) | Unirradiated unit (0) |
| AS-3 (–0.06) | AS-1 (0) |
| 60-year-old male recipient (0) | 85-year-old female recipient (+0.4) |
| BMI – 30 (–0.5) | BMI – 18 (-0.3) |
| Cumulative Hb increment: 0.59 g/dL | Cumulative Hb increment: 1.65 g/dL |
Simplified Model for Mean HEMOGLOBIN INCREMENTij = 2.41 + 0.1(MALE DONORij) − .06(DONOR/RECIPIENT RH NEGATIVEj) + 0.16(WHOLE BLOODij) + −0.06(SOLUTION AS 3ij) + −.08(UNIT GAMMA IRRADIATEDij) + −0.28(MALE RECIPIENTj) + 0.005(RECIPIENT AGEij)+ −.017(RECIPIENT BMIij) + −0.16(PRE-TRANSFUSION HB LEVELij)
Discussion
In this retrospective cohort study, the effects of blood donor, component, and recipient factors on hemoglobin increments after RBC transfusion were evaluated as a measure of transfusion efficacy. Overall, transfusion of a single unit of RBC resulted in an expected increase of 1 g/dL. However, combinations of donor, component, and recipient characteristics were responsible for a substantial proportion of the variation observed clinically. In addition to affirming clinically plausible recipient factors, this study provides relevant clinical practice data identifying measurable differences in hemoglobin increments related to donor sex, collection method, storage solution, and gamma irradiation of RBC. Collectively, these donor, component, and recipient factors could be used to estimate expected changes in hemoglobin levels after RBC transfusion.
Although not previously shown, findings of larger hemoglobin increments from transfusion of male-derived RBC units are not unexpected, given the higher RBC mass and content in 500-mL whole blood collections from male donors. However, in vitro studies have shown that RBCs from male donors and those exposed to testosterone exhibit increased susceptibility to spontaneous and stress-induced hemolysis after storage compared with female RBCs or exposure to female hormones.13 Detection of in vivo hemolysis of male-derived donor RBCs (as reflected by hemoglobin increment) may be masked by the higher hemoglobin content of these collections. RBC recovery and survival studies (with chromium-51 or biotin labeling) would obviate the need for future investigations to normalize for the hemoglobin dose of each RBC unit when assessing the impact of in vitro (during storage) and in vivo hemolysis.29,30
In parallel with donor sex, donor age is a characteristic that may have implications for transfusion outcomes.3,31 We found smaller hemoglobin increments for the RBC units transfused from the oldest group of donors. In older individuals, an inadequate rise in erythropoietin to compensate for aging marrow cells or loss of hematopoietic stem cell reserve may affect the red cell.32,-34 In addition, frequent blood donation has been associated with iron deficiency or iron-deficient erythropoiesis in older blood donors.35 Iron status in donors varies with age according to sex due to menstruation and pregnancy, and donor iron deficiency has been shown to affect posttransfusion recovery of RBCs in murine studies.36 Although measurable, differences related to donor age did not seem to have a clinically relevant effect on hemoglobin increments. Additional studies are needed to examine the relation between donor age, donation frequency, and storage duration on additional recipient outcomes.
Blood component manufacturing methods have been shown to influence in vitro characteristics of RBC products during storage, including hemoglobin content, biochemical properties, and rates of in vitro storage hemolysis.37,-39 Biochemical and immunomodulatory differences have been observed between units collected from whole blood and apheresis.8,15 For example, mitochondrial DNA release and extracellular vesicle damage-associated molecular patterns were increased in apheresis compared with whole blood–derived collections.14 We identified smaller hemoglobin increments in apheresis-derived units compared with whole blood–derived RBC collections. Higher RBC content in whole blood collections likely explains the larger hemoglobin increments; however, further investigation is needed to better understand these differences and other outcomes.
In the current cohort, gamma irradiation was associated with smaller hemoglobin increments compared with nonirradiated RBC units. Irradiation has been shown to increase potassium and free hemoglobin in the supernatant of RBC units through disruption of membrane integrity.16,40 Irradiated RBC units have also been shown to result in decreased recovery of RBCs after transfusion, particularly after longer durations of storage.41,42 In addition, a recent study found that in vitro storage of irradiated RBC units from female subjects had lower levels of hemolysis compared with male-derived RBCs.43 Based on our understanding of irradiation on in vitro RBC viability, it is relevant to show that gamma irradiation negatively affected hemoglobin increments. Accounting for potential selection bias in the receipt of an irradiated unit, we confirmed this finding in recipients with and without oncologic diagnoses. Interestingly, the effect of irradiation did not seem to be associated with the storage duration of the RBC product or with donor sex. Additional investigations are needed to correlate hemoglobin increments for irradiated units with in vivo measures of RBC hemolysis and other recipient outcomes.
The notion of the “red cell storage lesion” derives from a number of in vitro changes that occur during RBC storage, which may adversely affect RBC quality and function.17 The clinical significance of RBC storage has been studied in a series of randomized controlled trials (RCTs), which have not shown differences in morbidity and mortality outcomes when comparing fresh and stored RBCs.44,,,-48 The majority of these RCTs have not been powered to study the effect of RBCs stored beyond 35 days; in addition, these clinical trials did not examine associations of RBC storage on other measures of component efficacy.49,50 Similar to a recent study, we found that RBC storage duration beyond 35 days was associated with reduced hemoglobin increments after transfusion.51 This finding also parallels that of a prospective study of storage duration of autologous RBC units conducted in healthy volunteers that additionally detected increases in non–transferrin-bound iron and extravascular measures of hemolysis at the end of storage.18 Pending further RCTs, mechanistic clinical investigations and well-conducted observational studies provide us with the best evidence regarding the effectiveness and safety profile of 35- to 42-day-old RBC products.19,52
Reduced hemoglobin increments after selected RBC transfusion may reflect poor storage of blood components from specific donors with genetic polymorphisms.53 Genetic mutations associated with hemoglobinopathies, enzymopathies, and membranopathies have been postulated to affect RBC recovery during storage and after transfusion.54 Recently, the REDS-III RBC-Omics study found that RBCs from donors with Asian and African American ethnic backgrounds were associated with increased susceptibility to hemolysis after prolonged storage.12 Although the role of individual genetic polymorphisms may be small, combinations of heritable variants in relevant RBC genes in donors may substantially modulate in vitro hemolysis during storage and in vivo efficacy of RBC transfusions.55 Genome-wide association and metabolomic studies are in progress to further clarify these findings, including analysis of the outcomes of RBC transfusion episodes from the donors we present here.56,57 Also planned are mechanistic studies to determine if larger hemoglobin increments in Rh-D–positive donors and recipients have a genetic or metabolomic basis or if this finding represents residual confounding.58 Accounting for donor, component, and recipient factors documented in the current analysis will be critical to future studies examining the contribution of donor genetic polymorphisms on in vivo hemolysis and posttransfusion RBC recovery.
Today, transfusion services are increasingly adopting multidisciplinary patient blood management measures to provide fewer and more effective transfusions to achieve clinical outcomes, including adequate hemostasis. The role of individual recipient factors on hemoglobin increments after RBC transfusion has previously been described.59,-61 Increased increments for recipients with lower pretransfusion hemoglobin levels may reflect the relatively larger effect of the same RBC dose (∼89 g of hemoglobin per transfused unit) in patients with acutely reduced red cell mass or circulating blood volumes.62,-64 In this study, the difference in hemoglobin increments in sex-mismatched vs same sex donors and recipients was also striking. Male recipients of female-derived RBCs had significantly smaller increments than female recipients of male-derived units. Future studies should examine the role of hemoglobin increments and need for additional transfusion exposure when evaluating adverse outcomes associated with donor and transfusion recipient sex-mismatch. Furthermore, the variability in hemoglobin increments due to this and other factors (eg, component manufacturing methods) should be considered in the conduct of multicenter clinical trials of RBC transfusions, which frequently use multiple blood suppliers that collect, process, and store components differently.
In the current study, individual donor, component, and recipient characteristics played a small role in hemoglobin increments. However, collectively, they accounted for the significant variation observed in clinical practice and the substantial correlation between actual and predicted outcomes in regression model performance. Dramatically different increments after transfusion for the same pretransfusion hemoglobin level could affect clinical decision-making. For example, a suboptimal hemoglobin increment (eg, <0.8 g/dL) after transfusion may raise the question of ongoing bleeding or hemolysis, whereas a larger-than-expected increment may be falsely reassuring (Table 7). Prediction of hemoglobin increments using donor, component, and recipient data could also be used to optimize transfusion efficacy, especially in chronically transfused patients or resource-limited settings.
Results from research studies examining how biological factors in blood donors and component modifications contribute to the quality of RBC units will likely shape the way patients undergo transfusions in the future. Linked donor–component–recipient databases have the potential to validate laboratory or clinical trial findings. For example, differences in hemoglobin increments identified for RBCs stored in AS-3 correlated with in vitro and metabolomic findings, and they complement results of laboratory investigations of blood donor tobacco use on recipient outcomes.65,66 Given their sample size and longitudinal nature, linked databases may also be beneficial in providing safety and efficacy data for novel RBC products, processing, additive solutions, and pathogen reduction.67
The principal strength of the current study is the large linked donor–component–recipient cohort, which includes granular laboratory and clinical data as well as multivariate analysis to account for interactions and correlated events. Limitations of this study include analysis of recipients who received single-unit RBC transfusions in which the exact timing of hemoglobin levels could not be standardized. However, the overall strength of association of individual blood donor, component, and recipient factors did not vary significantly based on the timing of hemoglobin levels after transfusion. Future analyses accounting for volumes of IV fluids and other blood components concomitantly transfused with RBC units (which are more likely to occur with multiunit RBC episodes than with single-unit transfusions) are needed to assess for possible selection bias and show the generalizability of our findings. Studies focused on platelet and plasma transfusion could be valuable, as donor characteristics have not been well delineated for these products. The source of blood could be another relevant factor, as blood products transfused in this study were derived from a single blood supplier. In addition, details regarding blood-processing protocols and apheresis instruments were not available or standardized in our analysis. We are in the process of linking blood donor and component data, including details on collection and processing from other blood suppliers, to examine the role of different RBC manufacturing methods in our transfused recipient cohort. Subsequent analyses will need to account for donation frequency and other donor and component characteristics in adults but also focus on neonatal and pediatric populations.68 We chose hemoglobin increment as a measure of transfusion efficacy because hemoglobin levels frequently serve as the primary parameter in clinical decision-making for RBC transfusion.69,-71 However, future studies should examine other physiological biomarkers or outcomes to assess transfusion effectiveness and safety.44
In conclusion, we describe the association of blood donor characteristics, collection and modification methods, and recipient factors on hemoglobin increments related to RBC transfusion. Multivariate regression analysis revealed that individual donor, component, and recipient characteristics are significant in hemoglobin increments. Collectively, these factors account for much of the variation observed clinically and may have utility in predicting changes in hemoglobin with transfusion. Our findings support the ongoing need for large-scale evaluation of blood donor and component characteristics on recipient outcomes utilizing linked databases to further understand the risks and benefits of RBC transfusion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Jason Lee and the other members of the KPNC Blood Management Advisory Group for facilitating access to the blood bank data used in the study.
This study was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL126130).
Authorship
Contribution: N.H.R. had full access to the data in the study and takes responsibility for its integrity and the accuracy of the presented analyses; N.H.R., R.B., M.P.B., C.P., and G.J.E. were responsible for acquisition of data; C.P., C.L., and N.H.R. were responsible for the statistical analyses; M.P.B. and G.J.E. supplied administrative, technical, or material support; and all authors were responsible for study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nareg H. Roubinian, Kaiser Permanente Division of Research, 2000 Broadway, Oakland, CA 94612; e-mail: nareg.h.roubinian@kp.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal