Abstract
Transfusions of RBCs stored for longer durations are associated with adverse effects in hospitalized patients. We prospectively studied 14 healthy human volunteers who donated standard leuko-reduced, double RBC units. One unit was autologously transfused “fresh” (3-7 days of storage), and the other “older” unit was transfused after 40 to 42 days of storage. Of the routine laboratory parameters measured at defined times surrounding transfusion, significant differences between fresh and older transfusions were only observed in iron parameters and markers of extravascular hemolysis. Compared with fresh RBCs, mean serum total bilirubin increased by 0.55 mg/dL at 4 hours after transfusion of older RBCs (P = .0003), without significant changes in haptoglobin or lactate dehydrogenase. In addition, only after the older transfusion, transferrin saturation increased progressively over 4 hours to a mean of 64%, and non–transferrin-bound iron appeared, reaching a mean of 3.2μM. The increased concentrations of non–transferrin-bound iron correlated with enhanced proliferation in vitro of a pathogenic strain of Escherichia coli (r = 0.94, P = .002). Therefore, circulating non–transferrin-bound iron derived from rapid clearance of transfused, older stored RBCs may enhance transfusion-related complications, such as infection. The trial was registered with www.clinicaltrials.gov as #NCT01319552.
Introduction
The safety of transfusing RBCs after longer durations of refrigerated storage was recently identified as “the most critical issue facing transfusion medicine.”1 page 667 Concern was heightened when a large observational study of cardiac surgery patients found an increased risk of postoperative complications and reduced survival in those who received RBCs stored for more than 14 days.2 Although still controversial, adverse clinical consequences have since been reported in most,3-5 although not all,6,7 epidemiologic studies of transfusions of RBCs stored for longer durations, but still within Food and Drug Administration (FDA) guidelines. The association between the duration of RBC storage and increased rates of serious infections, sepsis, and mortality is particularly strong in trauma patients.7-11 Definitive determination of the potential risks associated with transfusion of RBCs stored for longer durations has been elusive, in part because the mechanisms responsible have not yet been identified.
More than 14 million RBC units are transfused in the United States each year, with a mean storage interval of 18 days before transfusion.12 During storage, RBCs undergo cumulative biochemical and biomechanical changes (the “storage lesion”) that reduce their survival in vivo after transfusion.13,14 In mouse models,15 transfusion of RBCs stored for longer durations was followed by brisk extravascular clearance of a subpopulation of these cells, which were damaged during storage and removed by macrophages in the spleen and liver of recipient mice. The iron liberated by phagocytic digestion of these RBCs rapidly entered the systemic circulation in amounts that exceeded the transport capacity of plasma transferrin, the physiologic iron-binding protein; in this way, circulating non–transferrin-bound iron appeared and promoted the proliferation of pathogenic bacteria both in vitro15 and in vivo.16
We hypothesized that the infectious complications observed in human patients after transfusion of RBCs stored for longer durations were, at least in part, the result of the production of circulating non–transferrin-bound iron. Therefore, we prospectively examined healthy human volunteers to determine (1) if transfusion of autologous RBCs stored for longer durations was followed by the appearance of circulating non–transferrin-bound iron in vivo, and (2) if this increased circulating non–transferrin-bound iron was associated with enhanced pathogenic bacterial growth in vitro.
Methods
Study design
Fourteen consecutive healthy adult volunteers were prospectively studied. Each volunteer donated, by a standard automated apheresis method (Alyx, Baxter Healthcare), a leuko reduced, double RBC unit, which was stored in a standard additive solution (Adsol/AS-1, Baxter Healthcare) in the Columbia University Medical Center–New York Presbyterian Hospital Blood Bank in compliance with FDA standards. Each volunteer was then transfused with 1 autologous RBC unit after 3 to 7 days of storage (ie, “fresh”) and subsequently with the second unit after 40 to 42 days of storage (ie, “older”). Timed blood samples were obtained 90 minutes before each transfusion and at 0, 1, 2, 4, 24, and 72 hours after transfusion. To prevent post-transfusion erythrocytosis and to maintain a parallel study design, a single-unit whole blood phlebotomy was performed 3 to 7 days before the transfusion of the older RBCs, if the volunteer's hemoglobin was more than 13.3 g/dL (Figure 1A). In addition, both fresh and older transfusions were started at approximately the same time of day (11:00 am), took place over approximately 2 hours at a rate of 150 mL/hour, and the same lunch was provided for both transfusion episodes at 12:00 noon. All transfusions were performed at Columbia University Medical Center–New York Presbyterian Hospital, and the double RBC units were collected and processed at the New York Blood Center. Study recruitment began in December 2008 and was completed by February 2011. The research protocol was approved by the Institutional Review Boards of both institutions and was conducted according to the principles expressed in the Declaration of Helsinki, and all participants provided written informed consent.
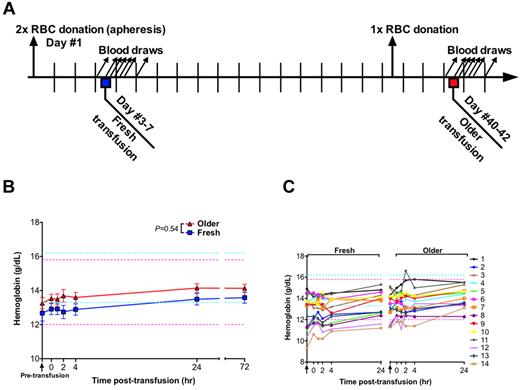
Study design and hemoglobin levels. (A) On day 1, each human volunteer donated 2 autologous RBC units by apheresis. One unit was transfused into the same participant after 3 to 7 days of storage (ie, “fresh”); the other unit was transfused after 40 to 42 days of storage (ie, “older”). One whole blood phlebotomy was performed 3 to 7 days before the older RBC transfusion to prevent post-transfusion erythrocytosis and to control for effects of recent blood loss on laboratory parameters. Blood samples were collected 90 minutes before transfusion and at 0, 1, 2, 4, 24, and 72 hours after transfusion. (B) Data are mean ± SEM for hemoglobin levels from before transfusion to 72 hours after transfusion of either fresh or older RBCs. The P value is as specified in the figure comparing the paired area under the curve of the mean hemoglobin levels for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions. (C) The individual hemoglobin levels for each subject up to 24 hours after transfusion. Vertical arrows indicate pretransfusion time points; and horizontal dashed lines, reference range values for men (blue) and women (pink).
Study design and hemoglobin levels. (A) On day 1, each human volunteer donated 2 autologous RBC units by apheresis. One unit was transfused into the same participant after 3 to 7 days of storage (ie, “fresh”); the other unit was transfused after 40 to 42 days of storage (ie, “older”). One whole blood phlebotomy was performed 3 to 7 days before the older RBC transfusion to prevent post-transfusion erythrocytosis and to control for effects of recent blood loss on laboratory parameters. Blood samples were collected 90 minutes before transfusion and at 0, 1, 2, 4, 24, and 72 hours after transfusion. (B) Data are mean ± SEM for hemoglobin levels from before transfusion to 72 hours after transfusion of either fresh or older RBCs. The P value is as specified in the figure comparing the paired area under the curve of the mean hemoglobin levels for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions. (C) The individual hemoglobin levels for each subject up to 24 hours after transfusion. Vertical arrows indicate pretransfusion time points; and horizontal dashed lines, reference range values for men (blue) and women (pink).
Study participants
The inclusion criteria were: healthy adults 18 to 65 years of age with male body weight > 59 kg (130 lbs), female body weight > 70 kg (155 lbs), male height > 1.55 m (5′1”), female height > 1.65 m (5′5”), and hemoglobin > 13.3 g/dL. Exclusion criteria were: ineligibility for donation based on the New York Blood Center autologous blood donor questionnaire, systolic blood pressure > 180 or < 90 mmHg, diastolic blood pressure > 100 or < 50 mmHg, heart rate < 50 beats/minute or > 100 beats/minute, temperature > 37.5°C before donation, temperature > 38°C, or subjective feeling of illness before transfusion, positive results on standard blood donor infectious disease testing, and pregnancy. All screened volunteers who met the inclusion criteria, and did not meet any of the exclusion criteria, were enrolled in the study.
Laboratory measurements
All laboratory testing for routine clinical parameters was performed in the Columbia University Medical Center–New York Presbyterian Hospital Clinical Laboratories. Non–transferrin-bound iron was measured using an ultrafiltration assay, as described,15 and was performed in the Iron Reference Laboratory at the Columbia University Medical Center. The reference range for plasma non–transferrin-bound iron in our laboratory is −0.71 to 0.10μM; data are presented as a change in non–transferrin-bound iron from pretransfusion levels. To eliminate interassay variability biasing the change in non–transferrin-bound iron levels, all the samples for a given volunteer were frozen at −80°C and were analyzed together after the final time point of study participation. IL-6 was measured with a high-sensitivity ELISA kit (R&D Systems) following the manufacturer's instructions.
Bacterial proliferation in vitro
Proliferation of a pathogenic strain of Escherichia coli, obtained from an anonymous patient with a urinary tract infection, was measured after inoculating all serum samples obtained from study participants, both before and after transfusion, as described.15 Briefly, 100-μL aliquots of serum in microtiter plate wells were incubated with 1 × 106 colony-forming units of E coli at 37°C with shaking. Optical density at 600 nm was measured periodically up to 5 hours after inoculation using a PowerWave XS microtiter plate reader (BioTek), and the area under the curve of the resultant growth curve was calculated using Prism 5 (GraphPad Software). All samples were inoculated in duplicate, and the mean of the 2 growth curves was used.
Statistical analysis
Differences in outcome measures after fresh or older RBC transfusions were compared using a paired t test or a Wilcoxon matched-pairs test, as appropriate, to compare the area under the curve of the increase in the outcome measure from 0 to 24 hours after transfusion. Normality of data was assessed using a D'Agostino and Pearson omnibus normality test. A P value of < .05 was considered significant. Statistical analyses were performed using Prism 5 (GraphPad Software). All data are presented as mean ± SEM, unless otherwise specified.
The study was originally powered for 11 participants, a sample size appropriate for detecting 15% of the non–transferrin-bound iron difference seen in mice, assuming the same SDs as observed in the mouse model.15 Recruitment was increased to 14 volunteers to include additional female participants, who were under-represented in the first 11 volunteers. Exclusion of these additional female volunteers from the analysis did not significantly change the results.
Results
Subject characteristics
Of 42 consecutive adults screened, 14 qualified for participation and all 14 volunteers completed the study with no dropout (study design shown in Figure 1A). There were no transfusion reactions and no significant changes in vital signs (blood pressure, heart rate, temperature) throughout the study. Two volunteers experienced lightheadedness during or immediately after donating blood, and 1 volunteer, who has chronic migraines, experienced a headache and vomited 2 hours after transfusion of fresh RBCs; the latter event was considered to be unrelated to study participation by both the study participant and the Data Safety Monitoring Board. Because of lower baseline hemoglobin levels (Table 1), only 1 of the 4 female participants met the hemoglobin criterion (> 13.3 g/dL) for the 1-unit whole blood phlebotomy 3 to 7 days before the older RBC transfusion. Exclusion of the 3 nonphlebotomized female participants from the analyses did not significantly change the results; thus, they were included in the analysis. All male participants met the hemoglobin criterion and were phlebotomized 1 whole blood unit before the older RBC transfusion.
Baseline characteristics of volunteers (N = 14)
| Characteristic . | Value . |
|---|---|
| Age, y (mean ± SD) | 30.4 ± 9.1 |
| Female sex, no. (%) | 4 (28.6) |
| Race/ethnicity,* no. (%) | |
| White | 9 (64) |
| Black | 1 (7) |
| Asian | 2 (14) |
| Hispanic | 2 (14) |
| ABO type, no. (%) | |
| A | 6 (43) |
| B | 5 (36) |
| O | 3 (21) |
| AB | 0 (0) |
| Height, m (mean ± SD) | 1.79 ± 0.09 |
| Weight, kg (mean ± SD) | 87.5 ± 17 |
| Hemoglobin before study, g/dL (mean ± SD) | |
| Male | 15.3 ± 1.2 |
| Female | 14.2 ± 0.8 |
| Characteristic . | Value . |
|---|---|
| Age, y (mean ± SD) | 30.4 ± 9.1 |
| Female sex, no. (%) | 4 (28.6) |
| Race/ethnicity,* no. (%) | |
| White | 9 (64) |
| Black | 1 (7) |
| Asian | 2 (14) |
| Hispanic | 2 (14) |
| ABO type, no. (%) | |
| A | 6 (43) |
| B | 5 (36) |
| O | 3 (21) |
| AB | 0 (0) |
| Height, m (mean ± SD) | 1.79 ± 0.09 |
| Weight, kg (mean ± SD) | 87.5 ± 17 |
| Hemoglobin before study, g/dL (mean ± SD) | |
| Male | 15.3 ± 1.2 |
| Female | 14.2 ± 0.8 |
Race/ethnicity was assessed by the investigators.
Effect of storage duration on complete blood counts
The mean pretransfusion hemoglobin (ie, after donating the RBC units, but 90 minutes before transfusion) was 12.7 and 13.3 g/dL, for the fresh and older RBC transfusions, respectively (P = .07 by paired t test). Given the volume of blood drawn for the timed samples, the hemoglobin was expected to increase by approximately 0.8 g/dL after transfusion. At 4 hours after transfusion, the mean hemoglobin increased by only 0.22 g/dL and 0.34 g/dL for the fresh and older transfusions, respectively. However, by 24 hours after transfusion, the mean hemoglobin increased by 0.82 and 0.89 g/dL for the fresh and older transfusions, respectively (P = .5442; Figure 1B-C). No significant differences between fresh and older transfusions were observed for white blood cell, absolute neutrophil, or platelet counts (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Effect of storage duration on basic metabolic parameters
There were no significant differences between fresh and older RBC transfusions in basic metabolic parameters (ie, sodium, potassium, chloride, blood urea nitrogen, creatinine, glucose, and total calcium; Figure 2; supplemental Figure 2). In particular, increased potassium levels were not observed after the older RBC transfusions (Figure 2A). However, both types of transfusions were associated with a progressive decrease in total calcium up to 4 hours after transfusion (Figure 2B), which was still evident after older RBC transfusions when the calcium levels were corrected for serum albumin levels (Figure 2C); ionized calcium was not measured.
Potassium levels do not change and calcium levels decrease after transfusions of older RBCs. The mean ± SEM for serum levels of (A) potassium, (B) total calcium, and (C) corrected calcium calculated as [(0.8 × (4.0 − subject's albumin)) + serum calcium]. The vertical arrow indicates the pretransfusion time point; and dotted lines, the reference ranges. The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions.
Potassium levels do not change and calcium levels decrease after transfusions of older RBCs. The mean ± SEM for serum levels of (A) potassium, (B) total calcium, and (C) corrected calcium calculated as [(0.8 × (4.0 − subject's albumin)) + serum calcium]. The vertical arrow indicates the pretransfusion time point; and dotted lines, the reference ranges. The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions.
Transfusion of older RBCs results in extravascular hemolysis
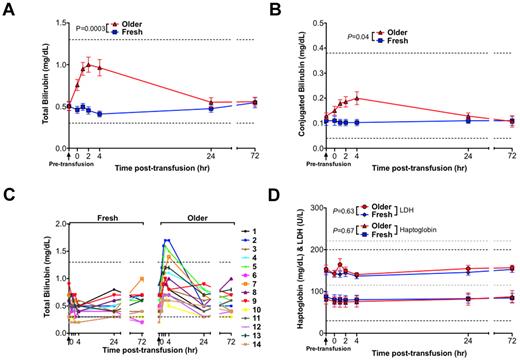
Compared with transfusions of fresh RBCs, transfusions of older RBCs were associated with significantly increased serum total bilirubin (P = .0003; Figure 3A), with a mean peak increase in total bilirubin of 0.55 mg/dL at 4 hours after transfusion. In addition, unconjugated bilirubin peaked above the reference range in 3 of 14 volunteers after transfusion of older RBCs (Figure 3C). Although the bilirubin was predominantly unconjugated, there was a small, but significant, rise in serum conjugated bilirubin (Figure 3D). No statistically significant differences between fresh and older RBC transfusions were observed in mean serum haptoglobin and lactate dehydrogenase levels, which are indicators of intravascular hemolysis (Figure 3D). There was a significant difference between transfusions of fresh and older RBCs in alanine aminotransferase levels (supplemental Figure 3; P = .01), although the difference was small (a 1.7-U/L increase 4 hours after older transfusions compared with fresh transfusions). There were no significant differences between fresh and older RBC transfusions in the other tested liver function parameters (ie, aspartate aminotransferase, alkaline phosphatase, total protein, and albumin; supplemental Figure 3).
Transfusions of older RBCs result in laboratory values consistent with extravascular hemolysis in healthy volunteers. Data are mean ± SEM for serum levels of (A) total bilirubin and (B) conjugated bilirubin from before transfusion to 72 hours after transfusion of both fresh and older RBCs. (C) The individual serum total bilirubin levels for all 14 volunteers from before transfusion to 72 hours after transfusion of both fresh and older RBCs. (D) Data are mean ± SEM for lactate dehydrogenase (LDH) and haptoglobin, from before transfusion to 72 hours after transfusion of both fresh and older RBCs. Although iatrogenic hemolysis was induced during a difficult blood draw for 2 volunteers at 1 hour after the older RBC transfusion, these samples were still included in the analysis; nonetheless, the analysis was not significantly altered by their exclusion. Vertical arrows in all panels indicate the pretransfusion time point; and dotted lines, the reference ranges (and in gray for LDH). The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older transfusions.
Transfusions of older RBCs result in laboratory values consistent with extravascular hemolysis in healthy volunteers. Data are mean ± SEM for serum levels of (A) total bilirubin and (B) conjugated bilirubin from before transfusion to 72 hours after transfusion of both fresh and older RBCs. (C) The individual serum total bilirubin levels for all 14 volunteers from before transfusion to 72 hours after transfusion of both fresh and older RBCs. (D) Data are mean ± SEM for lactate dehydrogenase (LDH) and haptoglobin, from before transfusion to 72 hours after transfusion of both fresh and older RBCs. Although iatrogenic hemolysis was induced during a difficult blood draw for 2 volunteers at 1 hour after the older RBC transfusion, these samples were still included in the analysis; nonetheless, the analysis was not significantly altered by their exclusion. Vertical arrows in all panels indicate the pretransfusion time point; and dotted lines, the reference ranges (and in gray for LDH). The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older transfusions.
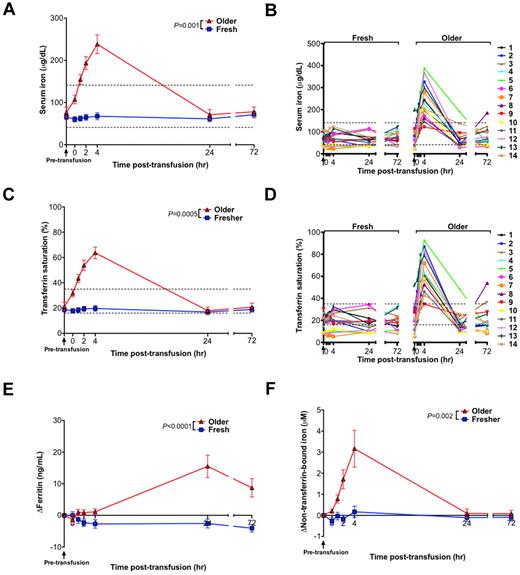
Transfusions of older RBCs increase iron parameters and produce circulating non–transferrin-bound iron
Although transfusions of fresh RBCs produced no significant change in mean serum iron or transferrin saturation, transfusions of older RBCs led to significant increases in serum iron (P = .001) and transferrin saturation (P = .0005), with a mean increase of 162 μg/dL and 42% over baseline, respectively, at 4 hours after transfusion (Figure 4A,C). In particular, serum iron and transferrin saturation peaked above the reference range in 13 of 14 volunteers after transfusion of older RBCs (Figure 4B,D). In addition, ferritin levels increased from the baseline pretransfusion sample only after transfusion of older RBCs, peaking at 15.5 ng/mL above baseline at 24 hours after transfusion (Figure 4E). Furthermore, after the fresh RBC transfusions, no significant increases in circulating non–transferrin-bound iron concentration were observed. In contrast, 13 of 14 volunteers had progressively increasing circulating non–transferrin-bound iron between 1 and 4 hours after transfusion of older RBCs reaching a mean of 3.2μM (P = .002) over baseline at 4 hours after transfusion (Figure 4F).
Iron parameters and circulating non–transferrin-bound iron levels increase after transfusions of older RBCs in healthy volunteers. (A) The mean ± SEM and (B) individual levels of serum iron; (C) mean ± SEM and (D) individual levels of transferrin saturation; (E) increase in ferritin compared with baseline levels; and (F) increase in plasma non–transferrin-bound iron compared with baseline levels from before transfusion to 72 hours after transfusion of fresh and older RBCs. Vertical arrows indicate pretransfusion time points; and dotted lines, the reference range (the reference range for change in ferritin and non–transferrin-bound iron is 0 by definition). The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions.
Iron parameters and circulating non–transferrin-bound iron levels increase after transfusions of older RBCs in healthy volunteers. (A) The mean ± SEM and (B) individual levels of serum iron; (C) mean ± SEM and (D) individual levels of transferrin saturation; (E) increase in ferritin compared with baseline levels; and (F) increase in plasma non–transferrin-bound iron compared with baseline levels from before transfusion to 72 hours after transfusion of fresh and older RBCs. Vertical arrows indicate pretransfusion time points; and dotted lines, the reference range (the reference range for change in ferritin and non–transferrin-bound iron is 0 by definition). The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions.
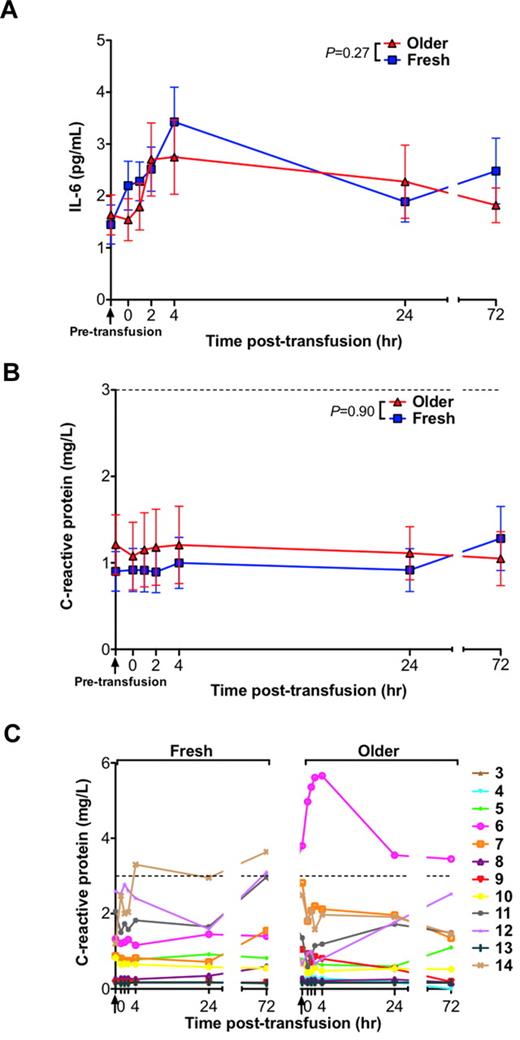
Effect of RBC storage duration on inflammation
Prior studies in mice demonstrated increases in various markers of inflammation after transfusions of older RBCs.15 However, in the current human study, there were no significant differences in IL-6 or C-reactive protein (CRP) levels between the groups receiving fresh and older RBC transfusions (Figure 5A-B). Nonetheless, 1 volunteer (number 14), had a CRP rise above the reference range between 4 and 72 hours after transfusion of only the fresh RBCs, and 1 volunteer (number 6), who had an elevated CRP level before the older RBC transfusion, manifested a progressive rise in CRP peaking 4 hours after transfusion of only the older RBCs (Figure 5C). Interestingly, in a post-hoc analysis, this volunteer was black with a Duffy-negative RBC antigen phenotype. Because the Duffy antigen is a chemokine receptor rarely absent on RBCs of persons of non-African descent, none of the other volunteers' RBCs were tested for the Duffy phenotype.
Serum levels of inflammatory markers do not increase after transfusions of older RBCs compared with fresh RBCs in healthy volunteers. (A) The mean ± SEM for serum IL-6 levels, (B) CRP levels, and (C) individual levels of CRP from before transfusion to 72 hours after transfusion of fresh and older RBCs. Vertical arrows indicate pretransfusion time points; and dotted lines, the reference range. The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers (for IL-6) and N = 12 (for CRP; the first 2 volunteers were not tested because of inadequate sample volume) from 0 to 24 hours after the fresh and older RBC transfusions.
Serum levels of inflammatory markers do not increase after transfusions of older RBCs compared with fresh RBCs in healthy volunteers. (A) The mean ± SEM for serum IL-6 levels, (B) CRP levels, and (C) individual levels of CRP from before transfusion to 72 hours after transfusion of fresh and older RBCs. Vertical arrows indicate pretransfusion time points; and dotted lines, the reference range. The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers (for IL-6) and N = 12 (for CRP; the first 2 volunteers were not tested because of inadequate sample volume) from 0 to 24 hours after the fresh and older RBC transfusions.
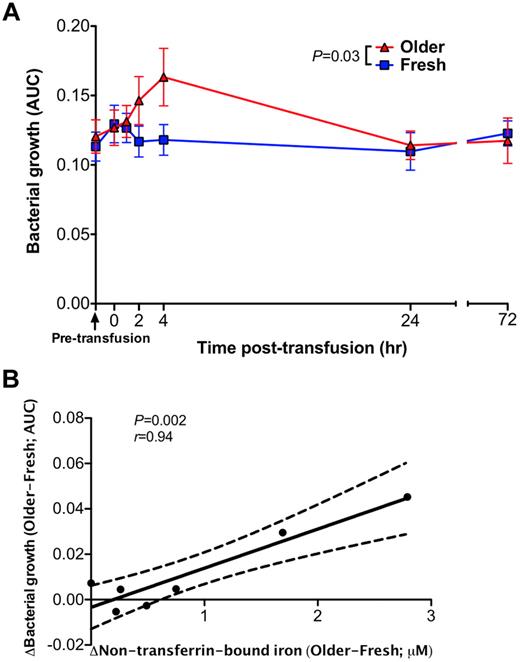
Transfusions of older RBCs enhance bacterial growth in vitro
A pathogenic strain of E coli obtained from an anonymous patient with a urinary tract infection was inoculated into all serum samples from all volunteers at all time points surrounding each transfusion. At 2 to 4 hours after transfusion of older (P = .03, Figure 6A), but not fresh, RBCs, the growth of E coli was enhanced in these serum samples. The mean difference in the area under the growth curve between fresh and older RBC transfusions correlated with the mean change in non–transferrin-bound iron (P = .002, Pearson r = 0.94; Figure 6B). Prior studies confirmed the iron-dependent growth of this bacterial isolate.15
Sera obtained after transfusions of older RBCs enhance proliferation of a bacterial pathogen in vitro. (A) Bacterial growth of E coli in serum samples obtained after fresh or older RBC transfusions was determined by serial optical density measurements at 600 nm for up to 5 hours after inoculation. Each point in the graph represents the mean ± SEM of the area under the curve (AUC) of the resultant bacterial growth curve (N = 14 paired values). (B) A Pearson correlation was used to determine the relationship between the mean difference in bacterial growth between fresh and older RBC transfusions at each time point and the corresponding differences in plasma non–transferrin-bound iron levels. The P values are as specified in the figure.
Sera obtained after transfusions of older RBCs enhance proliferation of a bacterial pathogen in vitro. (A) Bacterial growth of E coli in serum samples obtained after fresh or older RBC transfusions was determined by serial optical density measurements at 600 nm for up to 5 hours after inoculation. Each point in the graph represents the mean ± SEM of the area under the curve (AUC) of the resultant bacterial growth curve (N = 14 paired values). (B) A Pearson correlation was used to determine the relationship between the mean difference in bacterial growth between fresh and older RBC transfusions at each time point and the corresponding differences in plasma non–transferrin-bound iron levels. The P values are as specified in the figure.
Discussion
These results provide evidence of physiologic differences in the consequences of transfusions of RBCs after shorter (3-7 days) or longer (40-42 days) durations of storage, despite strict adherence to current FDA standards. Transfusions of fresh RBCs to 14 healthy volunteers produced no detected laboratory evidence of hemolysis and did not significantly alter serum iron, transferrin saturation, or circulating non–transferrin-bound iron. In contrast, despite appropriate increases in hemoglobin level, transfusions of older RBCs led to increased mean serum unconjugated bilirubin levels with no significant changes in mean serum haptoglobin or lactate dehydrogenase levels, a pattern consistent with rapid extravascular hemolysis of a subpopulation of the transfused older RBCs. Importantly, during the initial 4 hours after transfusion of older RBCs, serum iron and transferrin saturation increased significantly and circulating non–transferrin-bound iron appeared. These changes returned to baseline by 24 hours after transfusion. The potential pathogenic import of these differences was shown using a bacterial growth assay with these serum samples: increased proliferation in vitro of a pathogenic strain of E coli correlated with increased concentrations of non–transferrin-bound iron (r = 0.94, P = .002). Although no untoward clinical events occurred in these healthy volunteer recipients of older transfused RBCs, the potential for adverse infectious outcomes is conceivable, particularly for patients after cardiac surgery or trauma, who have open entry points for bacterial invasion and may be rapidly transfused with multiple units of RBCs of varying storage duration. However, further studies are necessary to extend these findings in serum in vitro to the clinical setting in vivo.
As RBCs age, while circulating in vivo or stored in vitro, they undergo changes that eventually lead to their recognition as senescent or damaged, and to their removal by macrophages in the spleen, bone marrow, and liver.15,17 In a typical healthy adult, approximately 1 mL of RBCs reaches the end of the life span and is cleared each hour, yielding approximately 1 mg of iron. This iron is either stored intracellularly or returned to the plasma to be bound by transferrin and transported to the erythroid marrow and other tissues for reuse. By current FDA standards, a unit of stored RBCs is acceptable for transfusion, even if 25% of the RBCs are cleared within 24 hours, an amount equivalent to as much as 60 mg of iron. Because most of this clearance takes place during the first hour after transfusion,18 the rate of delivery of heme-iron to reticuloendothelial macrophages may abruptly increase by as much as 60-fold after transfusion of even a single unit of packed RBCs. The corresponding accelerated rate of return of iron to plasma can surpass the rate of uptake by transferrin and produce circulating non–transferrin-bound iron.
In the current study, the kinetics of the appearance in the circulation of RBC degradation products (eg, bilirubin) demonstrate the rapid extravascular clearance of a subpopulation of the older transfused RBCs. Thus, although the mean hemoglobin increment after transfusion did not significantly differ between fresh and older RBC transfusions, the serum total bilirubin and transferrin saturation levels increased rapidly and in parallel during the initial 4 hours after transfusion of older stored RBCs (Figures 3–4). Despite the continued presence of unsaturated transferrin, circulating non–transferrin-bound iron appeared, probably the result of the rate of iron influx into plasma overwhelming the rate of iron acquisition by plasma transferrin.19 Because plasma non–transferrin-bound iron, a heterogeneous assortment of iron complexes,20,21 is available to pathogens reaching the bloodstream and can enhance their growth,22,23 we examined proliferation in vitro of a pathogenic strain of E coli. As shown in Figure 6A, serum samples obtained after transfusion of older RBCs significantly enhanced bacterial growth. Furthermore, as shown in Figure 6B, increased bacterial growth in serum obtained after transfusion of older, compared with fresher, RBCs closely correlated with the corresponding increases in circulating non–transferrin-bound iron. Because bacteria use various mechanisms for procuring iron,24 the contribution of non–transferrin-bound iron to virulence is expected to vary among organisms. Nonetheless, withholding iron from pathogens is a central component of host defense,23 and our results in vitro illustrate the capacity of circulating non–transferrin-bound iron to enhance infection. Interestingly, patients with transfusional iron overload, as well as those with hereditary forms of hemochromatosis, may have circulating non–transferrin-bound iron levels25,26 similar to those measured in our healthy volunteers at 4 hours after transfusion with older RBCs and are known to be at an increased risk for acute and chronic infections with specific pathogens.27-29 In addition, oral iron supplements are associated with transient increases in non–transferrin-bound iron,19,30 and routine supplementation with iron and folic acid in children in a malaria-endemic region increased the risk of severe illness and death.31,32
An incidental finding from this study is the extent of variability in hemoglobin levels measured soon after transfusion (Figure 1B-C), with 6 of 14 volunteers exhibiting either a decrease or no increase in hemoglobin at 4 hours after transfusion of fresh autologous RBCs. Although this finding supports classic textbook teaching33 that it requires up to 24 hours for hemoglobin levels to equilibrate, more recent studies suggest that hemoglobin levels quickly equilibrate after transfusion in adult34 and neonatal35 patients. Therefore, our findings suggest that hemoglobin measurements may not be ideal for assessing the effectiveness of RBC transfusions until 24 hours after transfusion, although this assertion is limited by an absence of measurements between 4 and 24 hours after transfusion. Decreases in serum albumin and total protein levels after transfusion of both fresh and older RBCs (supplemental Figure 3) suggest that there are significant volume shifts from the extravascular to the intravascular space after transfusion, which may help explain the observed variability in hemoglobin levels.
Contrary to the results in mouse studies,15 no difference in pro-inflammatory IL-6 levels was observed in healthy human volunteers after transfusion of fresh or older RBCs. Although mice may handle transfusion-induced iron loads differently than humans, the RBC dose transfused into humans may also have been too small and may have been given too slowly to elicit a pro-inflammatory cytokine response. For example, in the mouse studies, the rapid infusion (ie, “intravenous push”) of 2 RBC units elicited a robust pro-inflammatory cytokine response; in contrast, the human volunteers were transfused with only 1 RBC unit over a 2-hour time period.
Several limitations of this study should be taken into consideration. For example, only 1 unit was transfused over 2 hours per transfusion event; therefore, these results probably underestimate the effect on markers of hemolysis after transfusion in hospitalized patients who frequently receive multiple units at faster infusion rates. In addition, no blood samples were drawn between 4 and 24 hours after transfusion; therefore, the actual time intervals during which the iron parameters and markers of hemolysis remain elevated after transfusion of older RBCs are unknown. Still, these parameters predominantly return to baseline by 24 hours after transfusion, thereby indicating a relatively transient effect. Further studies are necessary to determine whether these transient effects are significant enough to affect the clinical course of transfused patients. Finally, there is an inherent time bias in the study design in that the older RBCs were always transfused approximately 35 days after the fresh blood transfusions.
These studies in healthy human volunteers and our related investigations in mice15,36 demonstrate that increased transferrin saturation leading to production of circulating non–transferrin-bound iron after transfusion of older RBCs is a potential mechanism for enhancing infectious complications in recipients. The concentrations of non–transferrin-bound iron observed after slow transfusion of a single unit of autologous RBCs in healthy human volunteers may be considerably lower than those found after rapid transfusion of multiple sequential units of allogeneic RBCs to severely ill trauma and surgical patients. Circulating non–transferrin-bound iron can also produce oxidative damage, thrombosis, cytotoxicity, and other types of injury15,20,37,38 and may contribute to additional mechanisms of increased morbidity and mortality after transfusions of older RBCs. Finally, other proposed mechanisms (eg, involving nitric oxide and/or microvesicles) may contribute to the increased morbidity and mortality that may result from transfusions of older RBCs.14,39-42 In conclusion, the physiologic differences described here in human volunteers after transfusion of fresh or older stored RBCs suggest that studies of novel blood storage systems, which improve post-transfusion RBC recovery, are warranted and that prospective clinical trials should determine whether transfusions of older RBCs enhance infectious and other disease risks in patients.
The online version of this article contains a data supplement.
Presented in part at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 6, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Inpatient and Outpatient Nurses and staff of the Irving Center for Clinical and Translational Research for their outstanding patient care and support of this study, Ms Ozaira Santana for administrative assistance, Dr Michael Shelanski for support and encouragement, and the human volunteers who took part in this study.
This work was supported by the National Institutes of Health (grants R01-HL098014, U01-HD064827, K08-HL103756), and a Louis V. Gerstner Scholars Award, as well as the National Center for Research Resources (grant UL1-RR024156), a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or National Institutes of Health.
National Institutes of Health
Authorship
Contribution: E.A.H., J.C.Z., J.E.H., G.M.B., and S.L.S. conceived the underlying model and designed the study; E.A.H., G.B.B., R.O.F., Y.Z.G., J.S., S. Sharma, S. Sheth, H.L.S., and B.A.S. acquired the data; E.A.H., G.M.B., G.B.B., R.O.F., J.J., A.N.S., S. Sharma, S. Sheth, B.A.S., B.S.W., and S.L.S. controlled and analyzed the data; E.A.H., G.B.B., J.J., J.S., and H.L.S. provided study supervision; E.A.H., G.M.B., and S.L.S. wrote the paper; and all authors edited drafts and reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eldad A. Hod, Department of Pathology and Cell Biology, 630 West 168th St, Room P&S 15-408, College of Physicians & Surgeons of Columbia University–New York Presbyterian Hospital, New York, NY 10032; e-mail: eh2217@columbia.edu.

![Figure 2. Potassium levels do not change and calcium levels decrease after transfusions of older RBCs. The mean ± SEM for serum levels of (A) potassium, (B) total calcium, and (C) corrected calcium calculated as [(0.8 × (4.0 − subject's albumin)) + serum calcium]. The vertical arrow indicates the pretransfusion time point; and dotted lines, the reference ranges. The P values are as specified in the figure comparing the paired area under the curve of the mean of the outcome parameter for the N = 14 volunteers from 0 to 24 hours after the fresh and older RBC transfusions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/25/10.1182_blood-2011-08-371849/4/m_zh89991183020002.jpeg?Expires=1769803454&Signature=cm69bg3VjV8CtxGTBoD-kyJqXM0DqIh6Oc882gfEIEgYBAnxSj~s2Qg5YzfkF4doaru~fG9Dk4tkONrun5SeC588x2QLXbbqurZejQJrvfwUX831lmY~0g5QfLaeuAYY71GVMNFlxAQZCSQ2gaYHajZwKbc6dpjd90d6rK9jtpbYzxykq1EYd2PtS9eGeKvHPTJQf2jrUWbWowELU9gxuTwgqC1-gZe7w60~FZpU6c9UK8pBHgHkv8pi0okfCMBr-dIngnS0pKP3QuKnph1aCJ2LT6LoxTlasimCMG4JNw-DS1APGXKQlzwJdTFqpGiKS96wULzYoY8ROzJ-u7JnYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal