Abstract
In patients undergoing percutaneous coronary intervention, catheter thrombosis is more frequent with fondaparinux than heparin. This study was undertaken to identify the responsible mechanism and to develop strategies for its prevention. Percutaneous coronary intervention catheter segments shortened plasma clotting times from 971 ± 92 to 352 ± 22 seconds. This activity is factor XII (fXII) dependent because it was attenuated with corn trypsin inhibitor and was abolished in fXII-deficient plasma. Heparin and enoxaparin blocked catheter-induced clotting at 0.5 and 2 anti-Xa U/mL, respectively, whereas fondaparinux had no effect. Addition of fondaparinux to bivalirudin or low-dose heparin attenuated catheter-induced clotting more than either agent alone. In a rabbit model of catheter thrombosis, a 70 anti-Xa U/kg intravenous bolus of heparin or enoxaparin prolonged the time to catheter occlusion by 4.6- and 2.5-fold, respectively, compared with saline, whereas the same dose of fondaparinux had no effect. Although 15 anti-Xa U/kg heparin had no effect on its own, when given in conjunction with 70 anti-Xa U/kg fondaparinux, the time to catheter occlusion was prolonged 2.9-fold. These findings indicate that (1) catheters are prothrombotic because they trigger fXII activation, and (2) fondaparinux does not prevent catheter-induced clotting unless supplemented with low-dose heparin or bivalirudin.
Introduction
In recent years, percutaneous coronary intervention (PCI) has emerged as routine therapy for acute coronary syndromes. Traditionally, unfractionated heparin has been the anticoagulant of choice to prevent thrombotic complications in patients with acute coronary syndrome undergoing PCI. During the past 10 years, however, there has been a shift to the use of smaller heparin fragments, starting with low-molecular-weight heparin (LMWH) and, more recently, with fondaparinux, a synthetic pentasaccharide.1,2 With better bioavailability, a longer half-life, and a more predictable anticoagulant response, LMWH and fondaparinux are more convenient than heparin because they can be given subcutaneously and they do not require coagulation monitoring.3-5 In addition, the risk of heparin-induced thrombocytopenia is lower with LMWH and fondaparinux than it is with heparin.6
Fondaparinux was compared with LMWH for treatment of patients with non–ST-segment elevation acute coronary syndrome and with heparin or placebo for management of patients with ST-segment elevation myocardial infarction.7,8 Although fondaparinux was found to be safe and effective in both trials, it was associated with an increased risk of guide catheter thrombosis in patients who underwent PCI.9 To explore the mechanism responsible for this phenomenon and to identify strategies to prevent it, we developed an in vitro plasma clotting assay to evaluate the extent to which PCI guide catheters activate clotting. Studies were then performed to (1) determine whether catheter-induced shortening of the clotting time reflects activation of the contact or extrinsic pathway of coagulation, (2) compare the capacities of heparin, enoxaparin and fondaparinux to inhibit catheter-induced clotting, and (3) examine the effect of supplemental heparin or bivalirudin on the capacity of fondaparinux to inhibit catheter-induced clotting. Finally, we used a model of accelerated catheter thrombosis in rabbits to determine whether our in vitro findings also apply in vivo.
Methods
Materials
Human plasmas deficient in factor VII (fVII), fXI, and fXII were obtained from Affinity Biologicals, whereas relipidated recombinant human tissue factor (RecombiPlasTin), was purchased from Hemoliance Instrumentation Laboratory. On the basis of immunoassay (American Diagnostica), the tissue factor concentration in RecombiPlasTin is 0.3 μg/mL. Corn trypsin inhibitor (CTI), bovine fXa and antithrombin, and human thrombin, fXIIa, fXIa, fIXa, and fXa were purchased from Enzyme Research Laboratories, Inc. Fondaparinux was from GlaxoSmithKline, enoxaparin was from Sanofi-Aventis Pharma Inc, and unfractionated heparin was from Leo Pharma Inc. Cyber guide catheters (6 Fr), composed of polyether block amide and polytetrafluoroethylene,10 with an outer diameter of 2 mm were a generous gift from Boston Scientific; 7 Fr polyurethane (PU) single-lumen catheters (Solo-Cath PU-C70) were purchased from Solomon Scientific.
Preparation of human platelet-poor plasma
After obtaining written informed consent, blood was collected from the antecubital veins of ≥ 10 healthy volunteers into 3.8% trisodium citrate (9:1 v/v) using a butterfly needle. The blood was maintained on ice until cellular elements were sedimented by centrifugation at 1500g for 20 minutes at 4°C. After removing the plasma and subjecting it to a second centrifugation step under the same conditions, the platelet-poor plasma was harvested, pooled, and frozen in aliquots at −80°C.
In vitro PCI catheter-induced clotting assay
PCI catheters were pressed flat with a hand roller and cut into 1.6-cm segments; a length chosen to fit the circumference of wells of 96-well polystyrene plates (Evergreen Scientific). Catheter segments were then shaped into rings and placed around the perimeter of wells, leaving the center of the well unobstructed. To the wells were added 75 μL of 20mM Tris-HCl, 150mM NaCl, pH 7.4 (TBS), and 100 μL of plasma. After 10 minutes of incubation at 37°C, clotting was initiated by addition of 25 μL of a prewarmed 160-mM CaCl2 solution (to yield a final CaCl2 concentration of 20mM), and clot formation was assessed by monitoring absorbance at 340 nm in kinetic mode with the use of a SPECTRAmax plate reader (Molecular Devices). Clotting times were defined as the time to one-half maximal absorbance and were calculated by instrument software from plots of absorbance versus time. Studies were repeated in plasma lacking fVII, fXI, or fXII. In some experiments, RecombiPlasTin was added to normal plasma to initiate clotting in wells that did or did not contain catheter segments. Where indicated, various concentrations of CTI, heparin, enoxaparin, or fondaparinux were incubated in plasma before recalcification. In subsequent experiments, fondaparinux was used in the absence or presence of heparin (at concentrations of 0.05 or 0.1 anti-Xa U/mL), bivalirudin (at concentrations of 12.5 or 50 μg/mL), or CTI (at a concentration of 100 μg/mL). The specific anti-Xa activities of heparin, enoxaparin, or fondaparinux used in these experiments were 180, 100, and 700 U/mg, respectively. Values for heparin and enoxaparin were provided by the suppliers, whereas, as previously reported, the specific anti-Xa activity of fondaparinux was obtained experimentally.11 Concentrations of bivalirudin were selected to match the plasma levels achieved with therapeutic doses of the drug.12
Comparison of the effect of heparin or fondaparinux on clotting induced by clotting enzymes in the extrinsic, contact, or common pathways of coagulation
Preliminary experiments were done to identify the concentrations of RecombiPlasTin, thrombin, fXa, fIXa, fXIa, or fXIIa that produced clotting times of ∼ 300 seconds, a value similar to that achieved with PCI catheter segments alone. A 1/1500-fold dilution of RecombiPlasTin, or thrombin, fXa, fIXa, fXIa, or fXIIa concentrations of 5, 0.13, 2.0, 0.5, or 20nM, respectively, yielded the requisite clotting times. For these experiments, 75 μL of TBS and 100 μL of plasma were added to wells of a 96-well plate in the absence or presence of various concentrations of heparin or fondaparinux. After incubation for 10 minutes at 37°C, clotting was initiated by addition of 25 μL of a 160-mM CaCl2 solution containing the requisite concentration of the various clotting enzymes. For thrombin-initiated reactions, CaCl2 was omitted to avoid feedback activation of coagulation, and thrombin was added after the 10-minute incubation. Clot times were determined by absorbance as described in “In vitro PCI catheter-induced clotting assay.”
Comparison of the effects of heparin, enoxaparin, or fondaparinux on the time to PU catheter occlusion in rabbits
A rabbit model of accelerated catheter thrombosis was developed by modifying the procedures of Du et al.13 Studies were approved by the Animal Research Ethics Board at McMaster University, and all procedures were in compliance with Canadian Council on Animal Care guidelines. Briefly, male New Zealand white rabbits (3-3.5 kg), purchased from Charles River Canada, were anaesthetized with a ketamine/xylazine mixture. PU catheters were cut into 15-cm segments and a 3.8-cm 16-gauge needle (reduced to 1.9 cm and with the end filed obliquely and then beveled) was inserted into one end. Catheters were flushed inside and outside with 10 mL of normal saline before a final flush with 1 mL of saline to remove any bubbles. After exposing the right jugular vein via a ventral incision, the catheter was inserted, advanced toward the heart for 7 cm, and secured with a ligature. With a syringe, 3 mL of blood was slowly withdrawn from the catheter, maintained within the syringe for 2 minutes, and then slowly re-injected. The catheter was then flushed with 2 mL of saline. This cycle was repeated every 5 minutes. A pressure transducer (Truwave Disposable Pressure Transducer; Baxter Healthcare Corp), placed between the catheter and the syringe, was used to quantify pressure within the catheter. The time to catheter occlusion was taken as the time when blood could no longer be withdrawn from the catheter, and the pressure within the catheter was > 15 mm Hg. At this point, or at 4 hours if catheter occlusion did not occur, the study was terminated, and 1000 U of heparin was injected via a central ear artery to prevent postmortem thrombosis, and the rabbits were killed with 1.5 mL of euthanyl.
Using this accelerated catheter thrombosis model, we first determined the time to catheter occlusion in rabbits (n = 5 per group) randomly assigned to receive saline or a 70 anti-Xa U/kg intravenous bolus of heparin, a dose within the recommended range for patients undergoing PCI,14,15 or an equivalent dose of enoxaparin or fondaparinux immediately before catheter insertion. Next, a dose-response study was performed with heparin to identify heparin doses that had little or no effect on the time to catheter occlusion. For this study, the time to catheter occlusion was compared in rabbits (n = 5 per group) randomly assigned to receive an intravenous bolus of either saline or heparin at doses of 15, 30, 50, or 70 anti-Xa U/kg. Building on the finding that a 15 anti-Xa U/kg dose of heparin had a minimal effect on the time to catheter occlusion, we then compared the time to catheter occlusion in rabbits (n = 5 per group) randomly assigned to receive an intravenous bolus of saline, 15 anti-Xa U/kg heparin, 70 anti-Xa U/kg fondaparinux, or the 2 agents in combination.
Blood sample analysis
Before catheter insertion and at intervals thereafter, 2-mL aliquots of blood were collected from a central ear vein into syringes prefilled with 0.2 mL of 3.8% citrate. After centrifugation at 1500g in a micro centrifuge, platelet-poor plasma was harvested, and anti-Xa activity was determined by chromogenic assay. Briefly, 2 μL of plasma was mixed with 88 μL of 50mM Tris-HCl, 150mM NaCl, pH 8, and containing 2.5mM EDTA. After incubation with 10 μL of a 5-μM bovine antithrombin solution for 4 minutes at 37°C, a 20-μL aliquot was removed, and 10 μL of 10nM bovine Xa was added and incubated for 30 seconds before the addition of 20 μL of 500μM S2765 (DiaPharma Group Inc). The amidolytic activity was then measured at 405 nm for 5 minutes with the use of a plate reader. An anti-Xa calibration curve was constructed in rabbit plasma containing known concentrations of heparin, enoxaparin, and fondaparinux. The specific anti-Xa activities of heparin, enoxaparin, and fondaparinux in rabbit plasma were 180, 100, and 300 U/mg, respectively. Values for heparin and enoxaparin are in agreement with those in human plasma provided by the suppliers, whereas, as previously reported, the specific anti-Xa activity of fondaparinux is 2-fold lower in rabbit plasma than in human plasma.16
Statistical analyses
Results are presented as the mean ± SD. Unless otherwise stated, experiments were performed ≥ 3 times. Comparisons of means of paired data were performed with paired Student t tests. To examine the effect of various concentrations of CTI on catheter-induced clotting times in human plasma, the clotting times were fitted to a linear model, and variations in slope were used to estimate the SD. Mean slopes were then compared with t tests. To compare the effects of various concentrations of heparin, enoxaparin, or fondaparinux on clotting times in human plasma, the clotting times were plotted versus the drug concentrations displayed on a log scale, a method that yielded a linear dose-response with each agent. The effects of the various anticoagulants on clotting times were then compared in terms of differences in slopes as determined by linear regression analysis. For all analyses, a P value < .05 was considered statistically significant.
Results
Effect of PCI catheter segments on plasma clotting times
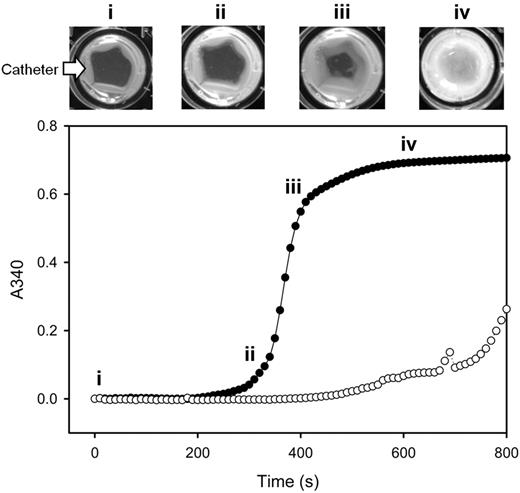
To explore the possibility that PCI catheters are prothrombotic, the clotting time of recalcified plasma was measured in a plate reader in the absence or presence of catheter segments. Catheter segments were placed around the perimeter of wells, and plasma clotting times were determined by monitoring absorbance. As shown in Figure 1, clot formation, evident as an increase in turbidity, started on the outside catheter surface and then extended toward the center of the well. The mean clotting time of normal plasma was 3-fold shorter in the presence of catheter segments than in their absence (352 ± 22 and 971 ± 92 seconds, respectively; P < .05; Table 1). Thus, PCI catheter segments exhibit prothrombotic activity, consistent with the observation that catheter thrombosis can occur in patients undergoing PCI.
Catheter-induced clotting in platelet-poor plasma. Pooled platelet-poor plasma was incubated in wells of a 96-well plate at 37°C in the absence (open circles) or presence of PCI catheter segments (closed circles). After the addition of CaCl2 to 20mM, absorbance was monitored at 340 nm at 10-second intervals, and the values were plotted versus time. A well from a separate plate at 37°C was photographed at 30-second intervals, and individual images at 4 time points (labeled i-iv) are displayed above the plot. The arrow identifies the catheter segment. With increasing incubation times, an opaque clot forms on the catheter segments positioned at the periphery of the well and then extends to fill the center of the well.
Catheter-induced clotting in platelet-poor plasma. Pooled platelet-poor plasma was incubated in wells of a 96-well plate at 37°C in the absence (open circles) or presence of PCI catheter segments (closed circles). After the addition of CaCl2 to 20mM, absorbance was monitored at 340 nm at 10-second intervals, and the values were plotted versus time. A well from a separate plate at 37°C was photographed at 30-second intervals, and individual images at 4 time points (labeled i-iv) are displayed above the plot. The arrow identifies the catheter segment. With increasing incubation times, an opaque clot forms on the catheter segments positioned at the periphery of the well and then extends to fill the center of the well.
The effect of catheter segments or tissue factor (RecombiPlasTin) on clotting times in normal or fVII-, fXI-, or fXII-deficient platelet-poor plasma
| Type of plasma/condition . | Time to clot, s . |
|---|---|
| Normal | |
| Control | 971 ± 92 |
| Catheter | 352 ± 22* |
| RecombiPlasTin | 102 ± 1* |
| Catheter + RecombiPlasTin | 93 ± 3* |
| fVII-deficient | |
| Control | 782 ± 35 |
| Catheter | 387 ± 27* |
| RecombiPlasTin | 514 ± 30* |
| Catheter + RecombiPlasTin | 320 ± 28* |
| fXI-deficient | |
| Control | > 2500 |
| Catheter | > 2500 |
| RecombiPlasTin | 136 ± 38* |
| Catheter + RecombiPlasTin | 140 ± 45* |
| f XII-deficient | |
| Control | 2467 ± 306 |
| Catheter | 1625 ± 532 |
| RecombiPlasTin | 133 ± 40* |
| Catheter + RecombiPlasTin | 131 ± 38* |
| Type of plasma/condition . | Time to clot, s . |
|---|---|
| Normal | |
| Control | 971 ± 92 |
| Catheter | 352 ± 22* |
| RecombiPlasTin | 102 ± 1* |
| Catheter + RecombiPlasTin | 93 ± 3* |
| fVII-deficient | |
| Control | 782 ± 35 |
| Catheter | 387 ± 27* |
| RecombiPlasTin | 514 ± 30* |
| Catheter + RecombiPlasTin | 320 ± 28* |
| fXI-deficient | |
| Control | > 2500 |
| Catheter | > 2500 |
| RecombiPlasTin | 136 ± 38* |
| Catheter + RecombiPlasTin | 140 ± 45* |
| f XII-deficient | |
| Control | 2467 ± 306 |
| Catheter | 1625 ± 532 |
| RecombiPlasTin | 133 ± 40* |
| Catheter + RecombiPlasTin | 131 ± 38* |
Plasma was incubated for 10 minutes at 37°C in the presence of PCI catheter segments before the addition of CaCl2 to 20mM with or without a 1/1500-fold dilution of RecombiPlasTin. As a control, clotting was monitored in the absence of catheters or RecombiPlasTin. Absorbance was monitored up to a maximum of 2500 seconds in a plate reader, and time to clot was determined as the time to one-half maximal absorbance. Results reflect the mean ± SD of ≥ 3 determinations.
P < .05 compared with the respective controls.
Determination of the coagulation pathway responsible for the prothrombotic activity of PCI catheter segments
Additional experiments were performed to determine whether PCI catheters exert their prothrombotic activity through activation of the extrinsic or contact pathway of coagulation. To explore involvement of the extrinsic pathway, 2 sets of experiments were performed. First, coagulation was initiated with RecombiPlasTin in the absence or presence of catheter segments. Catheter segments had no significant effect on RecombiPlasTin-induced clotting (Table 1), suggesting that the extrinsic pathway of coagulation is not affected by catheters. Second, the effect of catheter segments on the clotting time in normal plasma was compared with that in plasma deficient in fVII. No significant difference in clotting times was observed between normal plasma and plasma deficient in fVII in the presence of catheters, suggesting that catheter-induced activation of coagulation does not depend on fVII. Taken together, these findings suggest that the prothrombotic activity of PCI catheters does not reflect activation of the extrinsic pathway of coagulation.
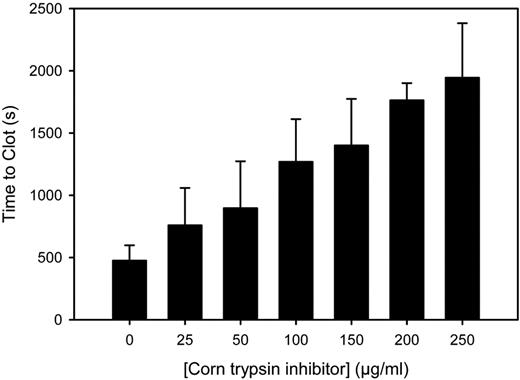
Next, studies were done to determine whether PCI catheters activate the contact pathway of coagulation. Compared with the control, CTI, a potent and specific inhibitor of fXIIa,17 had minimal effect on RecombiPlasTin-induced clotting (334 ± 18 and 322 ± 17 seconds, respectively; P = .46). In contrast, as shown in Figure 2, in the presence of catheter segments, CTI prolonged the clotting time, producing a significant (P = .005) linear increase in clotting time as a function of CTI concentration. These findings suggest that catheters trigger clotting via the contact pathway of coagulation and induce the generation of fXIIa. To further explore the role of this pathway, catheter-induced clotting in normal plasma was compared with that in plasma deficient in either fXII or fXI. The capacity of catheter segments to promote clotting was attenuated in both deficient plasmas (Table 1), suggesting that the prothrombotic effect of catheters depends on these 2 key components of the contact pathway. Thus, these studies show that PCI catheters exert their prothrombotic activity through the contact pathway of coagulation.
Effect of CTI on the prothrombotic activity of PCI catheter segments. Catheter segments were incubated with plasma for 10 minutes at 37°C in wells containing increasing concentrations of CTI. Clotting was initiated by the addition of CaCl2 to 20mM. Time to clot was determined as the time to reach one-half maximal absorbance. The bars represent the mean of ≥ 3 separate experiments, and the lines above the bars reflect the SD.
Effect of CTI on the prothrombotic activity of PCI catheter segments. Catheter segments were incubated with plasma for 10 minutes at 37°C in wells containing increasing concentrations of CTI. Clotting was initiated by the addition of CaCl2 to 20mM. Time to clot was determined as the time to reach one-half maximal absorbance. The bars represent the mean of ≥ 3 separate experiments, and the lines above the bars reflect the SD.
Effect of heparin, enoxaparin, or fondaparinux on PCI catheter segment-induced clotting in plasma
We next compared the effects of heparin, enoxaparin, or fondaparinux on catheter-induced clotting. As a control, the effect of these agents on RecombiPlasTin-induced clotting also was examined. As shown in Figure 3A, all 3 agents attenuated RecombiPlasTin-induced clotting in a concentration-dependent fashion, and clotting was attenuated to beyond 14 000 seconds with 0.5, 2, and 35 anti-Xa U/mL of heparin, enoxaparin, or fondaparinux, respectively. Comparisons of slopes obtained by linear regression analysis of plots of clotting time versus log-transformed drug concentrations yielded significant (P < .001) differences among the 3 agents (not shown). When catheters were used to induce clotting, heparin and enoxaparin again attenuated clotting in a concentration-dependent fashion (Figure 3B). In contrast, fondaparinux had minimal activity, prolonging clotting times only by a maximum of 2.1-fold at a concentration of 35 anti-Xa U/mL. Once again, comparison of slopes obtained by linear regression analysis of plots of log-transformed data yielded significant (P < .001) differences among the 3 agents. These data suggest that higher molecular weight heparin molecules attenuate catheter-induced clotting to a greater extent than those of lower molecular weight. The minimal effect of fondaparinux and the intermediate effect of enoxaparin in this system are consistent with the observation that catheter thrombosis occurred more frequently in fondaparinux-treated patients undergoing PCI than in patients given enoxaparin.8 To explore this phenomenon in more detail, the effects of heparin and fondaparinux on different steps in the coagulation cascade were examined.
Effect of fondaparinux, enoxaparin, or heparin on the prothrombotic activity of PCI catheter segments. Plasma was incubated with either (A) 1/1500 diluted RecombiPlasTin or (B) catheter segments, and the indicated concentrations of heparin (squares), enoxaparin (triangles), or fondaparinux (circles) for 10 minutes at 37°C. After initiating clotting by the addition of CaCl2, to 20mM, absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean of ≥ 3 separate experiments, and the lines above and below the symbols reflect the SD.
Effect of fondaparinux, enoxaparin, or heparin on the prothrombotic activity of PCI catheter segments. Plasma was incubated with either (A) 1/1500 diluted RecombiPlasTin or (B) catheter segments, and the indicated concentrations of heparin (squares), enoxaparin (triangles), or fondaparinux (circles) for 10 minutes at 37°C. After initiating clotting by the addition of CaCl2, to 20mM, absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean of ≥ 3 separate experiments, and the lines above and below the symbols reflect the SD.
Comparison of the effect of fondaparinux and heparin on clotting induced by RecombiPlasTin, thrombin or fXa, fIXa, fXla, or fXIIa
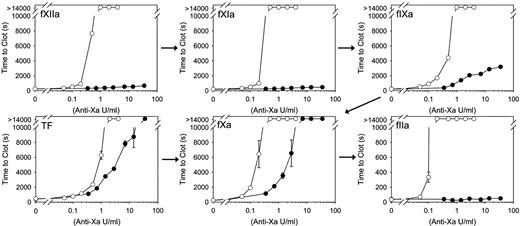
In contrast to its limited effect on catheter-induced clotting, fondaparinux prolongs the clotting time in a concentration-dependent fashion when dilute RecombiPlasTin is used to induce clotting. The distinct effects of fondaparinux on catheter- and RecombiPlasTin-induced clotting suggest that fondaparinux is limited in its capacity to inhibit clotting triggered via the contact pathway. To further explore this possibility, we compared the capacity of fondaparinux and heparin to inhibit clotting induced by fXIIa, fXIa, or fIXa. When clotting was triggered with fXIIa or fXIa, heparin prolonged the clotting time in a concentration-dependent fashion, and clotting was abrogated with a concentration of 1 anti-Xa U/mL (Figure 4). In contrast, fondaparinux, even at concentrations ≤ 70 anti-Xa U/mL, had no effect on clotting induced by fXIIa or fXIa. Heparin also inhibited clotting induced by fIXa in a concentration-dependent fashion and abrogated clotting at a concentration of 2 anti-Xa U/mL. Fondaparinux also prolonged the fIXa clotting time 5-fold at a concentration of 14 anti-Xa U/mL, consistent with the observation that fondaparinux promotes the inhibition of fIXa by antithrombin.18 When clotting was triggered with fXa, heparin and fondaparinux attenuated clotting in a concentration-dependent manner, and clotting was abrogated with concentrations of 0.5 and 7 anti-Xa U/mL, respectively. Whereas heparin inhibited thrombin-induced clotting in a concentration-dependent manner, fondaparinux had no effect, consistent with the concept that fondaparinux is too short to bridge antithrombin to thrombin. Overall, these results indicate that heparin is better than fondaparinux at inhibiting clotting induced by coagulation enzymes of the contact pathway. Although fondaparinux attenuates RecombiPlasTin and fXa-induced clotting, it has no effect on clotting triggered by thrombin, fXIa, or fXIIa and only limited effect on fIXa-induced clotting.
Effect of fondaparinux or heparin on clotting induced by RecombiPlasTin or coagulation enzymes. After incubating 1/1500-diluted RecombiPlasTin, 0.13nM fXa, 5nM fIXa, 0.5nM fXIa, or 20nM fXIIa in plasma containing fondaparinux (closed circles) or heparin (open circles), at the indicated concentrations, for 10 minutes at 37°C, clotting was initiated by addition of CaCl2 to 20mM. For thrombin-initiated reactions, CaCl2 was omitted to avoid feedback activation of coagulation, and thrombin (fIIa) was added to 5nM after the 10-minute incubation at 37°C. Absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean of ≥ 3 separate experiments, and the lines above and below the symbols reflect the SD. The arrows represent the contact, tissue factor, and common pathways of coagulation.
Effect of fondaparinux or heparin on clotting induced by RecombiPlasTin or coagulation enzymes. After incubating 1/1500-diluted RecombiPlasTin, 0.13nM fXa, 5nM fIXa, 0.5nM fXIa, or 20nM fXIIa in plasma containing fondaparinux (closed circles) or heparin (open circles), at the indicated concentrations, for 10 minutes at 37°C, clotting was initiated by addition of CaCl2 to 20mM. For thrombin-initiated reactions, CaCl2 was omitted to avoid feedback activation of coagulation, and thrombin (fIIa) was added to 5nM after the 10-minute incubation at 37°C. Absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean of ≥ 3 separate experiments, and the lines above and below the symbols reflect the SD. The arrows represent the contact, tissue factor, and common pathways of coagulation.
Effect of supplemental CTI, heparin, or bivalirudin on the ability of fondaparinux to attenuate PCI catheter-induced clotting
To further investigate the inability of fondaparinux to inhibit catheter-induced clotting, we examined the effect of supplemental CTI, heparin, or bivalirudin on the activity of fondaparinux. As expected, fondaparinux on its own had little or no effect on catheter-induced plasma clotting. In contrast, the addition of 100 μg/mL CTI (Figure 5A), 0.05 or 0.1 U/mL heparin (Figure 5B), or 12.5 or 50 μg/mL bivalirudin (Figure 5C) rendered fondaparinux more effective at attenuating catheter-induced clotting. Taken together, these findings suggest that low doses of heparin promote the antithrombotic activity of fondaparinux as do CTI or bivalirudin, anticoagulants that specifically target fXIIa or thrombin, respectively.
Effect of supplemental CTI, heparin, or bivalirudin on the capacity of fondaparinux to attenuate catheter-induced clotting. PCI catheter segments were incubated for 10 minutes at 37°C in plasma containing the indicated concentration of fondaparinux and (A) 0 (closed circles) or 100 (open circles) μg/mL CTI, (B) 0 (closed circles), 0.05 (open circles), or 0.1 (triangles) anti-Xa U/mL heparin, or (C) 0 (closed circles), 12.5 (open circles), or 50 (triangles) μg/mL bivalirudin. After initiating clotting by addition of CaCl2 to 20mM, absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean and the lines above and below the symbols reflect the SD.
Effect of supplemental CTI, heparin, or bivalirudin on the capacity of fondaparinux to attenuate catheter-induced clotting. PCI catheter segments were incubated for 10 minutes at 37°C in plasma containing the indicated concentration of fondaparinux and (A) 0 (closed circles) or 100 (open circles) μg/mL CTI, (B) 0 (closed circles), 0.05 (open circles), or 0.1 (triangles) anti-Xa U/mL heparin, or (C) 0 (closed circles), 12.5 (open circles), or 50 (triangles) μg/mL bivalirudin. After initiating clotting by addition of CaCl2 to 20mM, absorbance was monitored up to a maximum of 14 000 seconds, and time to clot was determined. The symbols represent the mean and the lines above and below the symbols reflect the SD.
Effect of heparin, enoxaparin, or fondaparinux on time to PU catheter occlusion in rabbits
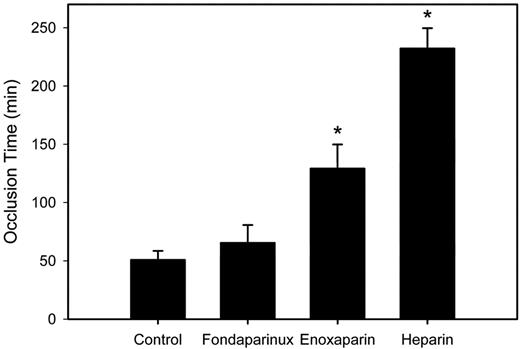
To determine whether our in vitro data also apply in vivo, we used a rabbit model of accelerated catheter thrombosis to compare the antithrombotic activities of heparin, enoxaparin, and fondaparinux. PU catheters were used in this model because PCI catheters lack the flexibility to maneuver the anatomy of the rabbit venous system. In the initial set of studies, rabbits were randomly assigned to receive saline or a 70 anti-Xa U/kg intravenous bolus of heparin, enoxaparin, or fondaparinux immediately before insertion of a PU catheter in the right atrium via the right jugular vein. In the control rabbits given saline, catheters occluded at 50.8 ± 7.6 minutes (Figure 6). The time to catheter occlusion was significantly (P < .05) prolonged by 4.6- or 2.5-fold in rabbits given heparin or enoxaparin, respectively, but was unchanged in those given fondaparinux. All 3 agents produced comparable levels of anticoagulation because mean plasma anti-Xa levels 5 minutes after drug administration administered were 1.0 ± 0.1, 1.0 ± 0.1, and 1.1 ± 0.1 U/mL, with fondaparinux, enoxaparin, and heparin, respectively (P = .93). Therefore, the disparate effects of these agents on the time to occlusion cannot be explained by differing anti-Xa levels. Taken together, the minimal effect of fondaparinux on the time to catheter occlusion and the intermediate effect of enoxaparin relative to heparin in the rabbit model are consistent with their activities in vitro.
Effect of fondaparinux, enoxaparin, or heparin on the time to PU catheter occlusion in rabbits. Rabbits (n = 5 per group) were given saline or 70 anti-Xa U/kg fondaparinux, enoxaparin, or heparin intravenously before insertion of a PU catheter into their jugular veins. Thereafter, every 5 minutes, 2 mL of blood was withdrawn from the catheter, held for 2 minutes in a syringe, and slowly re-injected. Catheter occlusion occurred when blood could no longer be withdrawn, and the pressure measured with a transducer exceeded 15 mm Hg. The bars represent the mean and the lines above the bars reflect the SD. Asterisks denote P < .05 compared with the saline control.
Effect of fondaparinux, enoxaparin, or heparin on the time to PU catheter occlusion in rabbits. Rabbits (n = 5 per group) were given saline or 70 anti-Xa U/kg fondaparinux, enoxaparin, or heparin intravenously before insertion of a PU catheter into their jugular veins. Thereafter, every 5 minutes, 2 mL of blood was withdrawn from the catheter, held for 2 minutes in a syringe, and slowly re-injected. Catheter occlusion occurred when blood could no longer be withdrawn, and the pressure measured with a transducer exceeded 15 mm Hg. The bars represent the mean and the lines above the bars reflect the SD. Asterisks denote P < .05 compared with the saline control.
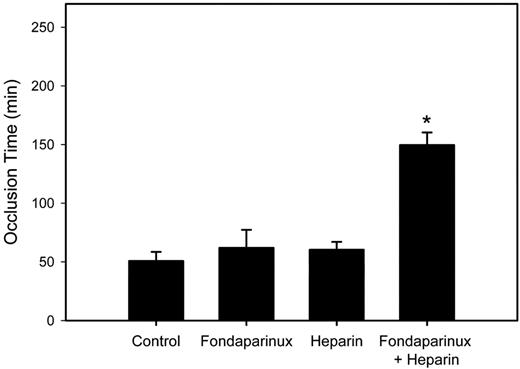
Next, we examined the effect of supplemental low-dose heparin on fondaparinux activity in the rabbit model. To identify a dose of supplemental heparin that has little or no activity, a pilot study was performed in which rabbits were randomly assigned to receive an intravenous bolus of either saline or heparin at doses of 15, 30, 50, or 70 anti-Xa U/kg. As before, a heparin dose of 70 anti-Xa U/kg prolonged the occlusion time to 4 hours (not shown). Whereas the 30 and 50 anti-Xa U/kg heparin doses had intermediate effects (prolonging the occlusion time from 50.8 ± 7.6 minutes to 80.4 ± 5.7 and 125.8 ± 17.8 minutes, respectively), with a heparin dose of 15 anti-Xa U/kg the mean time to occlusion of 60.4 ± 6.7 minutes was not significantly different from that in the saline-treated controls (P = .1). Building on this information, rabbits were next randomly assigned to receive an intravenous bolus of 70 anti-Xa U/kg fondaparinux, 15 anti-Xa U/kg heparin, or the 2 agents in combination. Control rabbits received an equal volume of saline. Although neither fondaparinux nor low-dose heparin alone had an effect on the time to occlusion, the combination produced a significant (P < .05) 2.9-fold prolongation of the mean time to occlusion compared with that in the saline control (Figure 7). These findings suggest that, like the results in vitro, supplemental low-dose heparin promotes the antithrombotic activity of fondaparinux in a more than additive fashion.
Effect of low-dose heparin alone or in conjunction with fondaparinux on the time to PU catheter occlusion in rabbits. Rabbits (n = 5 per group) were given an intravenous bolus of saline, 70 anti-Xa U/kg fondaparinux, 15 anti-Xa U/kg heparin, or the combination of 70 anti-Xa U/kg fondaparinux plus 15 anti-Xa U/kg heparin before insertion of a PU catheter into their jugular veins. The time to catheter occlusion was then determined as described in the caption to Figure 6. The bars represent the mean and the lines above the bars reflect the SD. Asterisk denotes P < .05 compared with the saline control.
Effect of low-dose heparin alone or in conjunction with fondaparinux on the time to PU catheter occlusion in rabbits. Rabbits (n = 5 per group) were given an intravenous bolus of saline, 70 anti-Xa U/kg fondaparinux, 15 anti-Xa U/kg heparin, or the combination of 70 anti-Xa U/kg fondaparinux plus 15 anti-Xa U/kg heparin before insertion of a PU catheter into their jugular veins. The time to catheter occlusion was then determined as described in the caption to Figure 6. The bars represent the mean and the lines above the bars reflect the SD. Asterisk denotes P < .05 compared with the saline control.
Discussion
Because of a reduced risk of bleeding,7 fondaparinux is an attractive alternative to enoxaparin for treatment of patients with non–ST-segment elevation acute coronary syndromes. However, the potential for catheter thrombosis in fondaparinux-treated patients who require PCI has dampened the enthusiasm for the use of this drug in patients who are managed invasively. To circumvent this problem, we set out to identify the mechanism responsible for catheter thrombosis with fondaparinux and to devise approaches for its prevention. Using in vitro and in vivo models, we demonstrated that (1) catheters are prothrombotic because of their propensity to activate fXII, thereby initiating the contact pathway of coagulation; (2) whereas heparin attenuates this phenomenon, fondaparinux is unable to inhibit catheter-induced clotting, and LMWH has an intermediate effect; and (3) supplemental bivalirudin or low-dose heparin promotes the capacity of fondaparinux to inhibit catheter-induced clotting, supporting the concept that adjunctive anticoagulation is useful for prevention of catheter thrombosis in fondaparinux-treated patients.
Two lines of evidence support the concept that the prothrombotic activity of catheters reflects the capacity to activate the contact pathway of coagulation. First, this activity was abolished in plasma deficient in fXII or fXI, key components of the contact pathway. Second, the addition of CTI, a specific fXIIa inhibitor, attenuated the prothrombotic properties of PCI catheter segments, consistent with the observation that blood-contacting medical devices adsorb and activate fXII.19 fXII is activated in the presence of dextran sulfate, polyphosphates, sulfatides, prolylcarboxypeptidase, DNA, and RNA.20-24 Numerous reports have shown that fXII can also be activated by synthetic materials such as polyurethanes, polytetrafluoroethylene, various polymers, and silicon.25-30 Catheters are often composed of such materials, which would explain why we observed similar procoagulant activity with coronary catheters and PU catheters obtained from different suppliers (not shown). Taken together, these results suggest that the contact pathway plays an important role in the initiation of catheter-induced clotting.
The correlation between our in vitro and in vivo findings and their concordance with the clinical trial results suggest that our in vitro assay provides a useful method to quantify the prothrombotic activity of catheters and to screen the capacity of various anticoagulants to abrogate catheter-induced clotting. These data also suggest that the inside and outside surfaces of catheters are equally prothrombotic and trigger clotting via similar mechanisms. Although thrombus on the inner surface of guide catheters is evident when the catheters are withdrawn, thrombus may also form on the outer surface. Likewise, the outside and inside surfaces of central venous catheters can trigger thrombosis. Thrombus on the outside surface of venous devices can lead to upper extremity deep vein thrombosis that can extend into the jugular vein and/or the superior vena cava, whereas thrombus formation on the inside surface of such devices can result in occlusion of the lumen and subsequent device failure.31 Therefore, our in vitro and in vivo models confirm the concept that catheters are prothrombotic and provide useful tools to investigate this phenomenon.
In contrast to heparin, fondaparinux was unable to inhibit catheter-induced clotting in vitro and in vivo, even at concentrations that far exceed those used clinically. Like heparin, enoxaparin inhibited catheter-induced clotting in vitro in a concentration-dependent fashion, but its effect was intermediate between that of heparin and fondaparinux both in vitro and in vivo. The disparate effects of heparin, enoxaparin, and fondaparinux in the rabbit model are unlikely to be the result of differences in the anti-Xa activities or half-lives of these agents because the peak anti-Xa levels with all 3 agents were the same, and fondaparinux and LMWH have longer half-lives than heparin.5 The inability of fondaparinux to prevent catheter thrombosis is in agreement with the results of a previous study, which showed clotting in catheters perfused with blood from fondaparinux-treated volunteers but not in catheters perfused with blood from heparin-treated subjects.32 The intermediate effect of enoxaparin in our studies is consistent with the results of the OASIS 5 trial,7 which showed catheter thrombosis in enoxaparin-treated patients, albeit at a lower rate than that observed in patients given fondaparinux, suggesting that enoxaparin does not eliminate this complication. Prevention of catheter thrombosis with enoxaparin requires an adequate dose of the drug. Because catheter thrombosis has been reported despite subcutaneous enoxaparin doses of 50 anti-Xa U/kg,33,34 most patients who are undergoing PCI are given intravenous enoxaparin at doses ranging from 75 to 100 anti-Xa U/kg.
Building on our observation that fondaparinux has limited inhibitory activity against proteases in the contact pathway, has no activity against thrombin, and is ineffective at preventing catheter-induced clotting, we examined whether the addition of agents that target the contact pathway and/or thrombin would render fondaparinux effective against catheter-induced clotting. Addition of a low dose of heparin to fondaparinux inhibited the prothrombotic activity of catheters both in vitro and in vivo. This reflects, at least in part, the capacity of heparin to inhibit thrombin because addition of bivalirudin, a specific thrombin inhibitor, with fondaparinux also attenuated the prothrombotic activity of catheters in plasma. In addition, however, heparin also inhibits contact pathway coagulation enzymes upstream of fXa, thereby attenuating fXa generation. Supporting the concept that attenuation of fXa generation through upstream inhibition contributes to the capacity of low-dose heparin to promote the activity of fondaparinux is the observation that combining CTI with fondaparinux also inhibited catheter-induced clotting in plasma. These findings raise the possibility that targeted inhibition of fXIIa or thrombin with CTI or bivalirudin, respectively, or adjunctive therapy with these agents or low-dose heparin may prevent catheter thrombosis in fondaparinux-treated patients.
In conclusion, we have shown in vitro and in vivo that catheters are prothrombotic because they activate the contact pathway of coagulation, and fondaparinux is unable to inhibit catheter-induced clotting because it does not inhibit upstream coagulation via the contact pathway nor does it inhibit downstream clotting mediated by thrombin. These findings suggest that (1) the potential for catheter thrombosis in fondaparinux-treated patients who require PCI can be largely overcome by administration of supplemental heparin, an approach that was used successfully in the FUTURA/OASIS 8 trial35 ; (2) fondaparinux may not be the best choice of anticoagulant for initial or extended treatment of patients with catheter-associated deep vein thrombosis because it is unable to inhibit catheter-induced clotting; and (3) because fXII activation is the root cause of catheter thrombosis, modifications of the catheter surface that limit this phenomenon may prevent catheter thrombosis, thereby obviating or attenuating the need for systemic anticoagulants.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported, in part, by the Canadian Institutes of Health Research (grants MOP 3992, MOP 102735, and CTP 79846), the Heart and Stroke Foundation of Ontario (grants T4729 and T4730), and the Ontario Research and Development Challenge Fund. J.I.W. is the recipient of the Heart and Stroke Foundation of Ontario/J. Fraser Mustard Endowed Chair in Cardiovascular Research and Canada Research Chair (Tier 1) in Thrombosis at McMaster University. Photography equipment and assistance was provided by Dr Howard Chan.
Authorship
Contribution: J.W.Y. designed research, performed research, analyzed and interpreted data, and wrote the manuscript; A.R.S. designed research, performed research, and analyzed and interpreted data; P.L. performed research and analyzed and interpreted data; J.C.F. designed research, analyzed and interpreted data, and wrote the manuscript; R.R. performed the statistical analyses; and J.I.W. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St E, Hamilton, ON, Canada L8L 2X2; e-mail: weitzj@taari.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal