In this issue of Blood, Lindahl et al report that aggressive antibiotic treatment inhibits disease activity and lymphocyte proliferation in cutaneous T-cell lymphoma (CTCL).1 These results are significant because they provide further evidence for a potential link between bacterial infection, activation of the immune system, and CTCL progression as well as a rationale for aggressive antibiotic treatment as adjuvant therapy in CTCL.
SA and its toxins activate STAT3 signaling and increase expression of the IL2R in tumor cells and nonmalignant T cells, thereby stimulating proliferation of tumor cells in CTCL. Antibiotic treatment can effectively eradicate this stimulus, normalize the tumor microenvironment, and inhibit disease activity in skin.
SA and its toxins activate STAT3 signaling and increase expression of the IL2R in tumor cells and nonmalignant T cells, thereby stimulating proliferation of tumor cells in CTCL. Antibiotic treatment can effectively eradicate this stimulus, normalize the tumor microenvironment, and inhibit disease activity in skin.
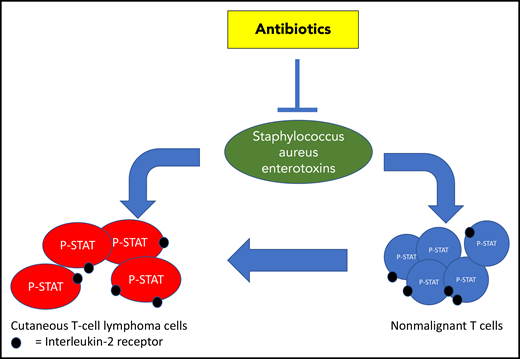
In recent years, next-generation sequencing studies have provided new insight into the genetic background of CTCL and identified, among others, STAT signaling as a critical pathway operative in CTCL tumor cells.2,3 A key question now is if interaction of tumor cells with their microenvironment can drive STAT signaling and thereby accelerate progression of disease. Biologic rationale for increased STAT signaling induced by bacterial products is most compelling. Patients with advanced stages of CTCL are frequently colonized with Staphylococcus aureus (SA), and antiseptic and antibiotic treatment can be helpful in clinical management.4 Malignant T cells may carry functional T-cell receptors (TCRs) expressing SA enterotoxin-binding Vβ chains, and SA enterotoxins may stimulate malignant T cells in vitro.5 Furthermore, in a STAT3-dependent mouse model of CTCL, disease progression was critically dependent on bacteria.6 In vitro studies showed that SA enterotoxins can stimulate a bidirectional cross talk between nonmalignant T cells and malignant CTCL cells that promotes proliferation of the malignant cells that depends on interleukin-2 (IL-2).7 Combined, these observations suggest that SA and its toxins may accelerate disease progression in CTCL either by directly stimulating tumor cells or by activating nonmalignant T cells, resulting in an inflammatory environment that may drive disease progression (see figure).
Following a dramatic clinical response on treatment with 4 weeks’ IV broad-spectrum antibiotics (carbapenem) in a mycosis fungoides patient (stage IIB) with extensive ulcerating tumors, Lindahl et al treated 8 additional patients with advanced refractory CTCL for 10 days with IV antibiotics (cephalosporin and metronidazole) and subsequent oral treatment for 14 days with combined amoxicillin and clavulanate.
Subjective improvement was observed as early as 10 days after initiation of antibiotic treatment, and in all patients, a marked clinical improvement with a significant decrease in skin disease burden was noted after 2 months. Immunohistochemical staining of skin biopsies taken before and 2 months after initiation of antibiotic treatment demonstrated that clinical improvement was accompanied by a decrease in cell proliferation, expression of interleukin-2 receptor (IL2R)-α, and tyrosine-phosphorylated STAT3 (pY-STAT3). Using TCRβ sequencing, it was found that the dominant TCR clonotype decreased significantly in 5 of 6 patients 60 days after initiation of antibiotic treatment, whereas the fraction of nonmalignant T cells increased following antibiotic therapy. Ex vivo studies showed that SA enterotoxins isolated from CTCL skin lesions could induce expression of pY-STAT3 and of the high-affinity IL2R-α chain in primary malignant cells and nonmalignant T cells. At transcription level, the clinical improvement was accompanied by a normalization of expression profiles with a clear decrease in IL-2 signaling and STAT activation. Importantly, antibiotics at clinically relevant concentrations did not induce apoptosis or affect the viability of malignant T cells in vitro. These observations suggest that SA and its toxins activate STAT3 signaling, increase expression of the IL2R, and stimulate proliferation of tumor cells in CTCL, whereas antibiotic treatment can effectively eradicate this stimulus, normalize the tumor microenvironment, and inhibit disease activity in skin.
Limitations of this study are the relatively small sample size and the short follow-up in the majority of patients. Therefore, it remains uncertain if the dramatic responses that were observed in this case series are a general characteristic in all CTCL patients and how durable these responses will prove to be.
A strength of this study is the translation of a clinical observation to a relatively small, but well-executed, clinical study combined with translational research that provides insight into the biology of the disease. Clearly, based on these observations, larger trials should be designed to optimize SA treatment protocols in the management of CTCL. Ideally, these studies should be combined with translational research to further explore the interaction of the microbiome with the immune system and malignant T cells. Insight into the cellular interactions, signaling pathways, and cytokines that play a role in the inflammatory microenvironment in CTCL may lead to identification of additional therapeutic targets. Because this study lends further support to the SA/STAT axis as a therapeutic target in CTCL, it will be of interest to explore synergy of antibiotics combined with STAT3 targeting treatment in these trials as well. As in the present study, all patients had advanced stages of disease, and open questions remain if similar interactions between microbiome, nonmalignant and malignant T cells are operative in early stages of CTCL as well and if manipulation of the skin microbiome in these early stages of disease can prevent disease progression.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal