By comparing fetal and adult B-lymphopoiesis, O’Byrne et al,1 in this issue of Blood, identified a pre-pro-B–cell subset that marks the earliest stages of B-cell lineage commitment in utero.
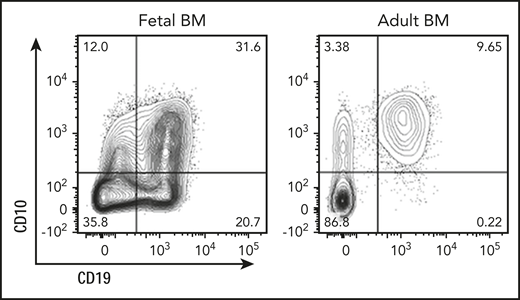
Fetal origin of human B-cell development. Pre-pro-B cells (CD19+CD10−CD34+) originate from the fetal liver and are abundant in fetal bone marrow (BM; shown here, left). In adult bone marrow, pre-pro-B cells are exceedingly rare (right) and differ from their fetal counterparts functionally and transcriptionally. See Figure 2A in the article by O’Byrne et al that begins on page 1059.
Fetal origin of human B-cell development. Pre-pro-B cells (CD19+CD10−CD34+) originate from the fetal liver and are abundant in fetal bone marrow (BM; shown here, left). In adult bone marrow, pre-pro-B cells are exceedingly rare (right) and differ from their fetal counterparts functionally and transcriptionally. See Figure 2A in the article by O’Byrne et al that begins on page 1059.
The origins and developmental progression of the B-cell lineage have been studied at substantial resolution for many years in mice.2,3 Many of the phenotypic and functional distinctions that demarcate early stages of B-cell development in mice are also applicable to differentiating B-cell subsets in adult human bone marrow.4 The pre-pro-B–cell subset, marking earliest stages of B-cell differentiation in mice, was discovered and functionally characterized 28 years ago5 ; however, developmental origins of human B-cell development remained elusive.
Although previous studies in humans focused on adult bone marrow,4 O’Byrne et al compared fetal liver and bone marrow to adult bone marrow samples. The authors identified pre-pro-B cells (CD19+CD10−CD34+) as the first committed B-cell precursor subset that lacks T-cell and myeloid potential, adding an important new observation to the emerging picture of human B-lymphopoiesis. Although abundant in fetal tissues, pre-pro-B cells are exceedingly rare in adult bone marrow (see figure) and differ from their fetal counterparts functionally and transcriptionally. Leveraging state-of-the-art technology, including single-cell RNA-seq, ATAC-seq for transcriptome profiling and to survey chromatin accessibility, O’Byrne et al reconstructed developmental trajectories that place the pre-pro-B–cell subsets downstream of early lymphoid progenitors (ELPs; CD19−CD10−CD34+CD127+) and upstream of pro-B cells (CD19+CD10+CD34+). Unlike ELPs, pre-pro-B cells did not retain non–B-lymphoid potential, suggesting that fetal pre-pro-B cells likely represent the earliest committed B-cell precursor. Despite the lack of T-cell and myeloid potential, pre-pro-B cells continued to express genes that are commonly expressed in hematopoietic stem or multipotent progenitor cells, myeloid, or T cells. Surface expression of CD19 represents the main phenotypic distinction between ELP and pre-pro-B cells. Given that CD19 expression requires activity of the PAX5 transcription factor,6 a central driver of B-cell lineage commitment,7 it is likely that initiation of B-cell lineage commitment mirrors the onset of PAX5 expression in pre-pro-B cells.
The existence of an even earlier B-lymphoid precursor population cannot be excluded, but it seems unlikely that these rare progenitors would be functionally and phenotypically distinct from ELPs and pre-pro-B cells in a meaningful way. Pre-pro-B cells and pro-B cells functionally overlap in that both subsets give rise to mature B-cell development and actively undergo V(D)J recombination of immunoglobulin VH region genes. Although D-JH rearrangements were detected in both pre-pro-B cells and pro-B cells, rearrangement of VH-to-DJH joints was specific for pro-B cells.
Studying matched liver and bone marrow samples from the same fetuses, the authors discovered 2 waves of early B-lymphopoiesis. Pre-pro-B cells first emerged in the fetal liver ∼7 weeks postconception, which was followed by a proliferative burst of pre-pro-B cells, mainly in fetal bone marrow. At 20 weeks postconception, pre-pro- and pro-B cells occupied nearly half of the hematopoietic progenitor cell pool in fetal bone marrow. The extent of proliferative expansion during these earliest stages of B-cell development was previously unrecognized and raised the possibility that pre-pro-B cells may be vulnerable to malignant transformation.
Adding to the significance of the discovery of this subset as developmental origin of the B-cell lineage, fetal pre-pro-B cells may also represent the cell of origin of acute lymphoblastic leukemia (B-ALL) developing in utero or early in postnatal life.8 For instance, B-ALL subtypes defined by MLL- or ETV6-RUNX1 rearrangements originate from a premalignant clone during fetal development.9 Like pre-pro-B cells, MLL-rearranged B-ALL clones typically carry immunoglobulin loci with rearranged D-JH but not VH-to-DJH segments and lack CD10 expression.10 Both pre-pro-B cells and MLL-rearranged B-ALL share a “mixed lineage” phenotype, although pre-pro-B cells, unlike MLL-rearranged B-ALL, lack myeloid lineage potential. Mirroring abundance of pre-pro-B cells in fetal bone marrow, MLL-rearranged B-ALL arises in utero and represents the vast majority of cases of infant B-ALL.9 In addition, O’Byrne et al highlight striking similarities in gene expression between fetal pre-pro-B cells and MLL-rearranged infant B-ALL.1 Based on a series of phenotypic and functional comparisons, the authors draw a fascinating connection between fetal pre-pro-B cells as the origin of human B-cell development and their potential role as cell of origin of MLL-rearranged infant B-ALL.
Conflict-of-interest disclosure: M.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal