In this issue of Blood, Goy et al report on the promising activity of a phase 1b trial of the targeted therapy triplet rituximab, ibrutinib, and lenalidomide in patients with relapsed nongerminal center diffuse large B-cell lymphoma (DLBCL).1
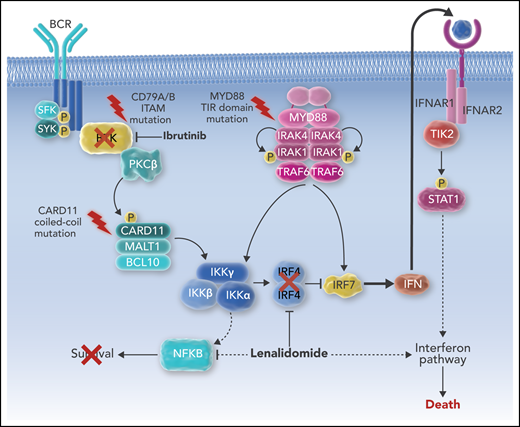
Ibrutinib and lenalidomide cooperate to kill ABC DLBCL via interferon signaling. P, phosphorylation.
Ibrutinib and lenalidomide cooperate to kill ABC DLBCL via interferon signaling. P, phosphorylation.
Approximately 2 of 3 patients with DLBCL, the most common lymphoid cancer, are cured with a chemotherapy combination originally created in 1976 (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]), which was last successfully modified in 1999 (by adding rituximab [R-CHOP]).2 For patients with DLBCL who are not cured by R-CHOP, a second chance for curative therapy is high-dose chemotherapy and autologous stem cell transplantation (ASCT). Patients who are not able to tolerate, do not respond to, or cannot access aggressive approaches such as ASCT or chimeric antigen receptor T-cell therapy have few therapeutic options with the potential for long-term disease control and are often considered palliative.3
DLBCL is a heterogeneous disease, most commonly classified on the basis of the putative cell of origin. The activated B-cell (ABC) subtype of DLBCL is characterized by chronic active B-cell receptor (BCR) signaling and requires NF-κB signaling for survival.4
The use of novel therapies that specifically target aberrant DLBCL biology has great promise, but it has yielded frustratingly few advances relevant to daily patient care to date. The BTK inhibitor ibrutinib is US Food and Drug Administration (FDA) approved for multiple B-cell malignancies and has been studied in DLBCL as a single agent, but responses were transient, with a median progression-free survival of 1.6 months and overall survival of 6.4 months.5 The immunomodulatory drug lenalidomide is FDA approved for multiple hematologic malignancies and has been studied as a single agent and in combination with rituximab in DLBCL patients, also demonstrating frustratingly short progression free survival of 2.7 and 2.8 months, respectively.6 Even more disappointing, both ibrutinib and lenalidomide have failed to significantly improve outcomes in DLBCL when added as single agents to R-CHOP.7,8 Why have these drugs with valid targets not resulted in better results?
Considering the many aberrances that cancer cells possess, perhaps their greatest asset is robustness. Drugs such as ibrutinib and lenalidomide have complex mechanisms of action, hitting many targets other than BTK or cereblon alone, including both tumor- and immune-mediated effects. Despite this pleiotropic potential, their potency as single agents (or as “+X” add-ons to standard chemotherapy) is limited because of the way cancer cells use alternate survival mechanisms to bypass their pathway blockade.9 To realize the true potential of targeted therapies, we must simultaneously target multiple related pathways to prevent escape.
The study of complex networks such as the internet, flight patterns, and cancer biology has found that this robustness is the result of a recurring pattern: a reliance on a few critical parts with redundancies that are difficult to damage simultaneously via nonspecific targeting.10 However, if critical parts of the network are concurrently targeted, the house of cards can collapse with limited effort.
The combination of ibrutinib and lenalidomide against DLBCL has been extensively studied in vitro, and a synthetic lethality was identified that was based upon interferon signaling in the ABC DLBCL subtype.11 In addition to antilymphoma immune changes, lenalidomide also decreases expression of IRF4, allowing for a modest amount of interferon production, which is toxic to ABC DLBCL. Ibrutinib, via blockade of BTK upstream in the BCR pathway, combines with lenalidomide to completely block IRF4 expression, resulting in an ABC DLBCL–lethal increase in interferon production (see figure). These two targeted agents, when administered simultaneously, are greater than the sum of their parts.
The multicenter phase 1b trial reported by Goy et al is impressive on the basis of its overall response rate of 38% (44% in evaluable patients), and a median duration of response of 15.9 months. In comparison with results for single agents, the Goy et al trial demonstrates 2 important points. First, these drugs can work very well in ABC DLBCL when used with the right partner, even if activity is only modest when used as a single agent or with the wrong partner (ie, chemotherapy). Second, the benefits of combination therapy, a hallmark of hematologic malignancy treatments for more than 40 years, applies in spades in the era of targeted therapies. Drug approval based upon single-agent activity (or as an add-on to chemotherapy) uses the imatinib paradigm of expecting miraculous single-agent results and can miss the full potential of active agents. Additional studies of rituximab-lenalidomide-ibrutinib and other targeted therapy combinations are warranted to improve the outcomes for our patients.
Conflict-of-interest disclosure: J.W. has received research support from and has consulted for Celgene and Janssen/Pharmacyclics for related research efforts but was not an investigator on the study by Goy et al.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal