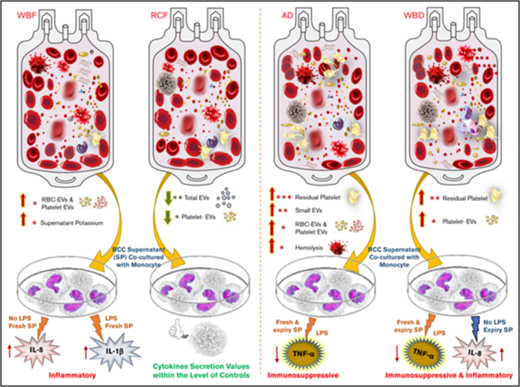
Key Points
EVs vary by RCC manufacturing method; among methods tested, red cell filtered RCCs had lowest total and cell-specific EVs.
Manufacturing method affects immune activity of RCC supernatants: AD RCCs were immunosuppressive; WBF RCCs were inflammatory.
Abstract
Transfusion of red cell concentrates (RCCs) is associated with increased risk of adverse outcomes that may be affected by different blood manufacturing methods and the presence of extracellular vesicles (EVs). We investigated the effect of different manufacturing methods on hemolysis, residual cells, cell-derived EVs, and immunomodulatory effects on monocyte activity. Thirty-two RCC units produced using whole blood filtration (WBF), red cell filtration (RCF), apheresis-derived (AD), and whole blood–derived (WBD) methods were examined (n = 8 per method). Residual platelet and white blood cells (WBCs) and the concentration, cell of origin, and characterization of EVs in RCC supernatants were assessed in fresh and stored supernatants. Immunomodulatory activity of RCC supernatants was assessed by quantifying monocyte cytokine production capacity in an in vitro transfusion model. RCF units yielded the lowest number of platelet and WBC-derived EVs, whereas the highest number of platelet EVs was in AD (day 5) and in WBD (day 42). The number of small EVs (<200 nm) was greater than large EVs (≥200 nm) in all tested supernatants, and the highest level of small EVs were in AD units. Immunomodulatory activity was mixed, with evidence of both inflammatory and immunosuppressive effects. Monocytes produced more inflammatory interleukin-8 after exposure to fresh WBF or expired WBD supernatants. Exposure to supernatants from AD and WBD RCC suppressed monocyte lipopolysaccharide-induced cytokine production. Manufacturing methods significantly affect RCC unit EV characteristics and are associated with an immunomodulatory effect of RCC supernatants, which may affect the quality and safety of RCCs.
Introduction
Red blood cell (RBC) transfusion remains common, particularly in critically ill patients.1-3 However, transfusion of red cell concentrates (RCCs) is independently associated with increased risks of nosocomial infection, organ dysfunction, and death.4-6 Transfusion-related immunomodulation (TRIM) includes both immunosuppressive and inflammatory effects that may in part explain increased risks in patients who receive blood transfusions.7-11 Mechanisms of adverse effects related to red cell transfusion remain uncertain, although RCC contain a host of biologically active mediators, in both soluble and cell-associated forms, which may contribute to organ dysfunction via alterations in recipient inflammation and immune cell function.7,12-14 Although many previous studies have focused on accumulation of potentially harmful immunomodulatory mediators during RCC storage,8,15,16 recent randomized clinical trials have failed to demonstrate benefit with fresh RCC transfusion in critically ill or hospitalized patients,17 thus calling into question the clinical relevance of storage-related TRIM effects. It has been suggested that RCC manufacturing methods, which are rarely accounted for in interventional trials, may have confounded these results.18,19 Differences in blood component manufacturing methods and RCC characteristics across clinical trial sites may mask the effect of RCC storage duration on patient outcomes.18,19 Indeed, in a large Canadian registry study comparing the whole blood filtration method to the red cell filtration method for RCC product preparation, transfusion with fresh whole blood filtered (WBF) red cells was independently associated with in-hospital mortality.20 Differences in blood component manufacturing methods may result in significant differences in potential immunomodulatory mediators, such as intracellular factors released by hemolysis, residual platelets and leukocytes, and extracellular vesicles (EVs), and may play a significant role in posttransfusion immunomodulatory effects.21-24
In addition, the presence of EVs in RCC products is an important factor that has emerged as a potential mediator of the immunomodulatory activity posttransfusion.25-27 EVs, heterogeneous submicron-sized vesicles, are produced and released by many types of cells.26,28 However, most studies do not take into account the heterogeneity of EVs in RCCs in terms of the size, phenotype/cell of origin, composition, and surface biomarkers. Because these EVs, which accumulate in RCC during storage, can differ in terms of their biogenesis and biophysical properties29 and can be influenced by different blood manufacturing methods, their immunomodulatory activity may vary as well. Thus, the aim of this study was to investigate the effect of different manufacturing methods on RCC characteristics, including hemolysis, residual cell counts, and extracellular vesicles; and on the immunomodulatory activity of RCC supernatants on monocyte function.
Methods
Blood collection and manufacturing
All blood donors provided signed, informed consent at the time of donation. Whole blood was collected from healthy donors, and RCC (n = 32) were produced using 4 different blood manufacturing methods30 (8 units per method). WBF and red cell filtered (RCF) RCC were collected by the Canadian Blood Services, whereas apheresis-derived (AD) and whole blood–derived (WBD) RCC were collected by Blood Systems in the United States.
Whole blood filtration method.
Whole blood was collected into blood collection sets (DQE 7292LX, Leucoflex MTL1 quadruple Top/Top system, MacoPharma) with 70 mL citrate-phosphate-dextrose (CPD) anticoagulant and processed using the whole blood filtration method (n = 8). After collection, whole blood was cooled and leukoreduced by filtration in the refrigerator within 48 hours of stop-bleeding time. Filtered units were then centrifuged at 4552g for 6 minutes to separate the blood components. An automated extractor (Compomat G4, Fresenius-Kabi) was used to extract plasma; saline-adenine-glucose-mannitol was added to RCC.
Red cell filtration method.
Whole blood was collected into blood collection sets (LQT 7292LX Leucoflex LCR-Diamond quadruple Top/Bottom system, MacoPharma) with 70 mL of CPD anticoagulant and processed using the RCF method (n = 8). After collection, units were rapidly cooled to 18°C to 24°C and held overnight. Products were then centrifuged at 3493g for 11 minutes and separated into the blood components (plasma, RBC, and buffy coat) using an automated blood-processing device (Compomat G4). Then, saline-adenine-glucose-mannitol was added to the extracted RCC. The RCC units were leukoreduced by filtration at room temperature within 24 hours of stop-bleeding time.
Apheresis-derived methods.
RCC collected using apheresis cell separators (Trima Accel Apheresis System, Terumo BCT; software 6.0.6; Trima Accel 80500 kit) with 70 mL of anticoagulant citrate dextrose solution, solution A (ACD-A) and 200 mL additive solution (AS-3). After collection, RCC units were filtered at room temperature.
Whole blood derived method.
Whole blood was collected into blood collection sets (Fenwal 4R1587P Flex Triple, WB 500 mL) with 70 mL of CPD anticoagulant. WB units were centrifuged at 5895g for 8 minutes at 1°C to 6°C. Plasma was extracted, the RCC was retained in the original bag, and 110 mL of AS-1 was added.
Shipping, storage, and sampling.
Using packing configurations designed to maintain RCC at an appropriate temperature (1°C to 10°C), RCC units were shipped to the Canadian Blood Services laboratory in Edmonton, AB, Canada. All shipments arrived within 24 hours of being packed and RCC were stored between 1°C and 6°C in a monitored refrigerator for up to 42 days. RCC sampling (25% of the unit volume) was performed once on day 5 (fresh) and once on day 42 (expired) postcollection as previously described.31,32 An aliquot (5 mL) of each day 5 sample was set for residual cell counting and in vitro quality parameter testing. The remaining RCC samples were centrifuged at 1000g for 10 minutes at 4°C (Eppendorf 5810R) to separate cells from supernatant. Supernatant was collected and transferred to cryovials and frozen at ≤65°C. One frozen supernatant aliquot from each unit (fresh and expired) was used at Canadian Blood Services for in vitro quality assessments and to measure EV concentration and size profile by qNano. Additional frozen supernatant aliquots from each unit (fresh and expired) were shipped on dry ice to 2 centers for additional analyses: (1) Blood Systems Research Institute (San Francisco, CA) to test the cell of origin of EVs by flow cytometry and (2) The Research Institute at Nationwide Children's Hospital (Columbus, OH) for monocyte coculture testing. All testing was performed on the day 5 and day 42 aliquots, except residual cell counts, which were measured on day 5 supernatants only (supplemental Figure 2).
In vitro quality assessment of RCC units
Hemolysis was determined using a Drabkin-based spectrophotometric method as previously described.32-34 Briefly, for hematocrit (Hct), RCC were aspirated into self-sealing Hct capillary tubes and read visually after centrifugation for 5 minutes in a Hct centrifuge (Hettich Haematokrit Centrifuge Type 2010). Total hemoglobin and supernatant hemoglobin were treated with Drabkin reagent and measured spectrophotometrically using a microplate reader (SpectraMax 384 Plus, Molecular Devices Corp.). Percent hemolysis was determined using Hct and measured values for supernatant hemoglobin and total hemoglobin as previously described.21 Supernatant samples were sent to an accredited laboratory (Alberta Health Services) for analysis on an automated chemistry analyzer (DXC800, Beckman Coulter, Inc) to measure supernatant potassium concentrations as described previously.32
Residual cell counts
RBC samples (1 mL) were sent to the Canadian Blood Services National Testing Laboratory (Ottawa, ON, Canada) to determine residual white blood cell (WBC) levels using flow cytometry as previously described.22 Residual platelet counts were also measured by the flow cytometer using lineage-specific monoclonal antibodies as described previously,23,24 with some modifications. Briefly, RCC (100 μL) were diluted with buffer (1× phosphate-buffered saline) and 5 μL of the fluorescently labeled monoclonal antibodies (PerCP/Cy5.5 anti-human CD41a antibody; BD Biosciences, Mississauga, ON) were added to identify platelets. Commercial isotype control (PerCP/Cy5.5 mouse IgG1, isotype control; BD Biosciences) was used as a negative control. After 15 minutes of incubation in the dark at room temperature, prepared samples were run on a bench-top digital flow cytometer (LSR-Fortessa X-20, BD Biosciences) with TruCOUNT beads (BD Biosciences) used to determine the absolute number of platelets per microliter. Results were analyzed using BD FACSDiva 8.0.1 software (BD Biosciences).
Extracellular vesicle characterization
QNano assay for extracellular vesicle concentration and size profiling.
Quantification and size characterization of EVs in RCC were measured using a tunable resistive pulse sensing instrument (TRPS/qNano system; IZON Science Ltd) as previously described in detail.35,36 Two different nanopores (NP200 and NP400) were used in this study to target EVs <1 µm in size using a standard stretch range (43-47 mm). Carboxylate polystyrene calibration particles (CPC200; IZON Science Ltd) were used with the NP200 to characterized EVs <200 nm in diameter, whereas CPC500 (IZON Science Ltd) was used with NP400 nanopore to calibrate for EVs ≥200 nm. Supernatant samples were diluted with electrolyte solution (RK1 measurement electrolyte, IZON Reagent kit) and the sample dilution adjusted as required to target a particle rate of 1000 to 2000/min. Samples were filtered with Millex syringe filter (Merck Millipore Ltd) before being analyzed with NP400 or NP200, as recommended by the manufacturer. Samples and calibration particle measurements were run under the same conditions and at least 1000 particles were recorded with 2 different standard pressure ranges (1 unit = 1 mbar). Data obtained were analyzed using IZON Control Suite software, version 3.3.
Flow cytometry assay for extracellular vesicle phenotyping and quantification.
EV phenotyping was performed using a flow cytometer as previously described.37,38 Briefly, 20 μL of the supernatant of each RCC product was stained with the following linage-specific monoclonal antibodies to identify the cell of origin of EVs: CD41a-PerCP-Cy5.5, CD142-APC, CD66b-PE, CD144-BV421, CD235a-FITC, CD3-FITC, and CD14-PE-Cy7 (BioLegend), and CD16-ECD, CD19-PerCP-Cy5.5, and CD62P-APC (BD Biosciences). Stained samples were incubated in the dark for 30 minutes at room temperature, diluted in 1× phosphate-buffered saline, and acquired on an LSRII flow cytometer for 60 seconds (BD Biosciences); sufficient events were collected to provide approximately ≥5000 gated EV events. An AbC Anti-Mouse Bead Kit (Life Technologies) was used to set the compensation along with the single-stained compensation control. Small beads ranging from 0.2 to 1 μm (Megmix-Plus SSC beads, Biocytex) were used to generate the EV gate and to further classify them based on their size (only EVs ≤1.0 µm in diameter were analyzed). BD TruCOUNT tubes (BD Biosciences) were used to obtain the absolute number of EVs/μL. Data were analyzed using FlowJo v10.
Monocyte coculture experiment
Monocyte in vitro transfusion model and cytokine measurements.
Monocytes were isolated from whole blood of 8 healthy adult donors as previously described39,40 and were used immediately in coculture models. The monocyte coculture model was adapted from our previously published in vitro transfusion model.39,40 For each experimental replicate, 1 × 106 healthy adult monocytes were plated on 12-well tissue culture plates in complete tissue culture media with 20% by volume RCC supernatants or complete tissue culture media only as control for 4 hours at 37°C in 5% CO2 incubator. The 20% by volume of RCC supernatant was chosen to approximate the volume ratio of a 20 mL/kg RBC transfusion. After this incubation, cells were stimulated with 1 ng/mL of lipopolysaccharides (LPS) from Salmonella enterica serotype abortus equi (Sigma) for 4 hours. Cell supernatants from each well were collected and stored at −80°C for batch analysis of LPS-induced cytokines. Pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-8, and the anti-inflammatory cytokine IL-10 were quantified by chemiluminescence using the IMMULITE 1000 automated chemiluminometer (Siemens Healthcare Diagnostics). All experimental replicates were performed using different healthy adult monocyte donors and different RCC units. Endotoxin and pyrogen-free reagents and labware were used for all experiments.
Statistical analysis
For the monocyte coculture experiment, comparisons between RCC product groups were analyzed using analysis of variance with Dunnett posttest for multiple comparisons. All statistical analyses were performed using Prism 7.00 (GraphPad Inc). For EV characterization, statistical analysis was completed using SPSS (IBM SPSS Statistics 23.0). Analysis of variance followed by a Tukey post hoc test was used to identify significant differences within the storage period for EV assays and to evaluate any significance among pairwise comparisons of testing time points during the storage time. Paired Student t tests were used to identify significant differences between the testing time points (days 7 and 42). Pearson correlation coefficient and associated P value were calculated between EVs and cytokines for all of the RCC units and for each blood manufacturing method. Linear model analysis was performed to test the significant of the correlations between the manufacturing methods. P < .05 was considered significant throughout the study.
Results
In vitro quality parameters
Residual cell count.
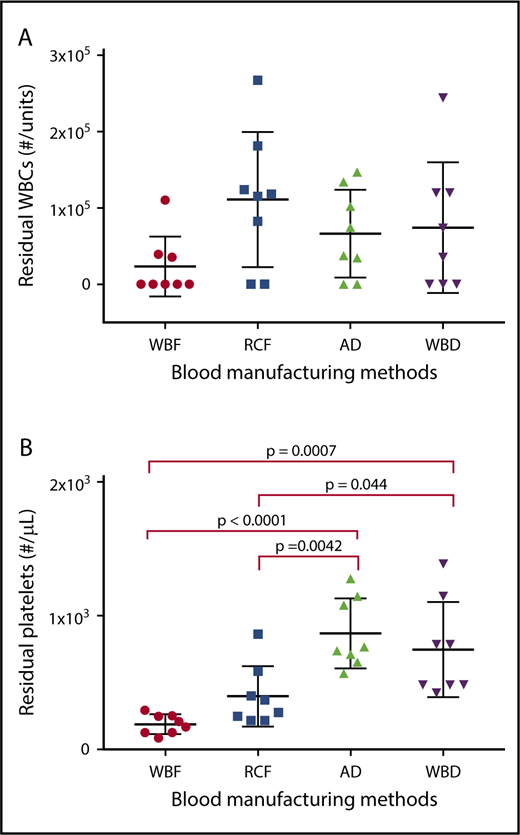
Although all of the blood manufacturing methods had similar level of residual WBC, quantities of residual platelets differed among the products based on the processing methods used (Figure 1).
Residual WBC and platelet counts in RCC produced by different manufacturing methods as measured on day 5 of storage. Data reported for (A) residual white blood cells and (B) residual platelets as scatter dot plots with mean and standard deviation..
Residual WBC and platelet counts in RCC produced by different manufacturing methods as measured on day 5 of storage. Data reported for (A) residual white blood cells and (B) residual platelets as scatter dot plots with mean and standard deviation..
Supernatant potassium and percent hemolysis.
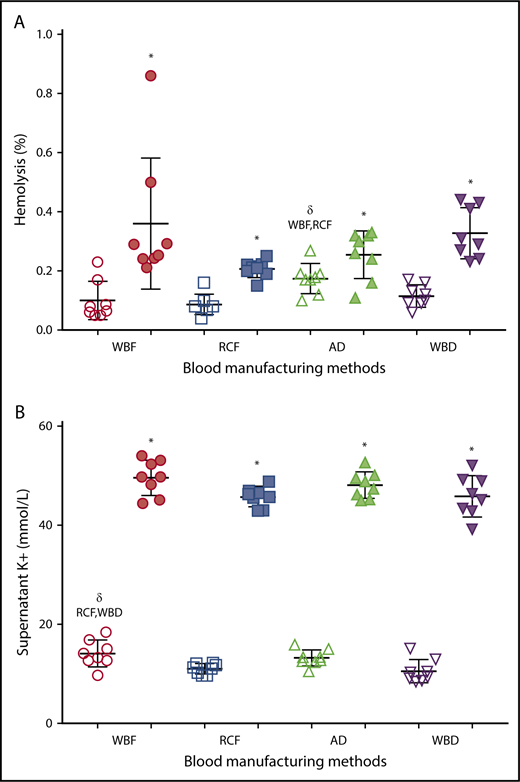
As expected, there was a significant increase in hemolysis during storage in all of the RCC products (Figure 2A), with no significant differences among manufacturing methods of the expired units. However among day 5 units, AD RCCs demonstrated greater hemolysis compared with RCF (P = .006) and WBF (P = .025) units. Supernatant potassium also increased over storage time in all of the RCC products, with no significant differences among manufacturing methods at day 42 (Figure 2B). On day 5 of storage, supernatant potassium was significantly higher in WBF units compared with RCF (P = .024) and WBD (P = .008) RCCs (Figure 2B).
Hemolysis and supernatant K+of differently manufactured RCC products. Dot plots display (A) percent hemolysis and (B) level of supernatant K+ on day 5 (fresh/white) and day 42 (expired/shaded) of stored and differently manufactured RCC products. Data reported as scatter dot plots with mean and standard deviation. *Significant results (P < .05) in comparison with day 5 values. δSignificant difference (P < .05) compared with the noted blood manufacturing methods.
Hemolysis and supernatant K+of differently manufactured RCC products. Dot plots display (A) percent hemolysis and (B) level of supernatant K+ on day 5 (fresh/white) and day 42 (expired/shaded) of stored and differently manufactured RCC products. Data reported as scatter dot plots with mean and standard deviation. *Significant results (P < .05) in comparison with day 5 values. δSignificant difference (P < .05) compared with the noted blood manufacturing methods.
Characterization of EV populations by tunable resistive pulse sensing
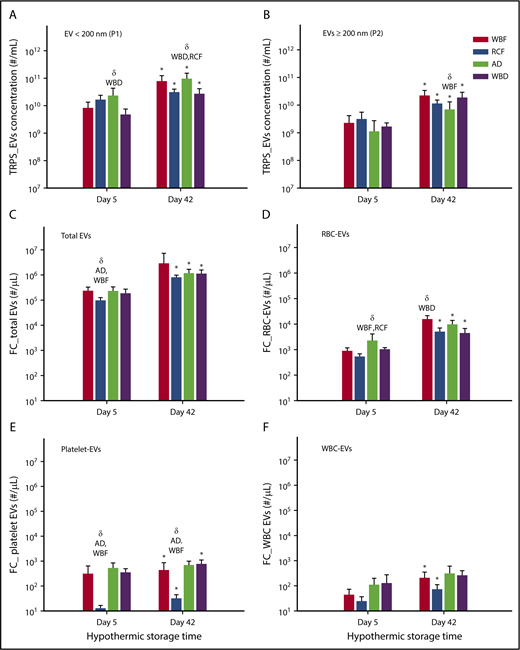
There was an increase in the total number of EVs (EVs per milliliter) on day 42 in comparison with day 5 of storage in all blood manufacturing methods. In addition, the number of small EVs/exosomes (<200 nm) was greater than large EVs (≥200 nm) in all of the products on days 5 and 42 (Figure 3A-B). Notably, the highest level of EVs <200 nm was in AD units, which were significantly different from WBD on day 5 (P = .0115) as well as WBD and RCF on day 42 (P = .0050 and P = .0083, respectively; Figure 3A). No statistically significant differences among the blood products was observed with larger EVs (EVs ≥200 nm) except on day 42 between AD and WBF RCC (P = .0106, Figure 3B). Furthermore, the size profile of EV showed significant differences in the EV size-profile among all RBC products (P < .05). On day 5 of storage, WBF-RCC had a different EVs size profile (smaller EVs, 91.1 ± 6.8 nm) in comparison with apheresis-RCC (125.4 ± 37.0 nm; P = .009). On day 42 of storage, the mean of small EVs (<200 nm) apheresis and WBF RCC was lower compared with RCF and WBD products (P < .05) (data not shown).
Concentration of EV and subpopulation in RCC products stored for up to 42 days analyzed by the TRPS and flow cytometry systems. (A) EV < 200 nm, (B) EV ≥ 200 nm, (C) total EV, (D) RBC-EV, (E) platelet-EV, and (F) WBC-EV. Data are reported as mean ± standard deviation. *Significant results (P < .05) in comparison with day 5 values. δSignificant difference (P < .05) compared with the noted blood manufacturing methods (n = 8 per blood manufacturing method).
Concentration of EV and subpopulation in RCC products stored for up to 42 days analyzed by the TRPS and flow cytometry systems. (A) EV < 200 nm, (B) EV ≥ 200 nm, (C) total EV, (D) RBC-EV, (E) platelet-EV, and (F) WBC-EV. Data are reported as mean ± standard deviation. *Significant results (P < .05) in comparison with day 5 values. δSignificant difference (P < .05) compared with the noted blood manufacturing methods (n = 8 per blood manufacturing method).
EV quantification and cells of origin by flow cytometry
Across all groups, EV counts measured by flow cytometry were orders of magnitude lower than those measured by TRPS, suggesting that flow cytometric analyses may have missed some of the smaller EVs. Consistent with the TRPS data, flow cytometry results showed a significant increase in the number of total EVs (EVs/µL) on day 42 of hypothermic storage in all of blood manufacturing methods (P < .05) compared with day 5 (Figure 3C). Among day 5 supernatants, RCF units had the lowest total EV and platelet-derived EV concentrations (Figure 3C,E), whereas AD units had the highest RBC-derived EV concentrations (Figure 3D). Among day 42 supernatants, RBC-derived EV concentrations were highest in WBD supernatants (Figure 3D), whereas RCF supernatants again demonstrated the lowest concentration of platelet-derived EVs (Figure 3E).
Monocyte coculture
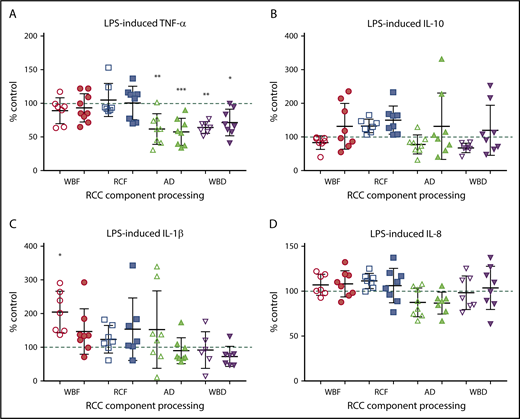
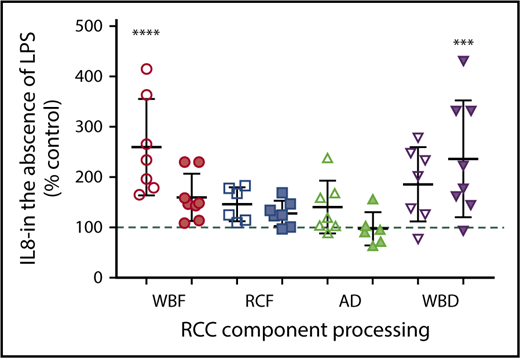
The immunomodulatory effects of RCC supernatants on monocytes were mixed and differed by manufacturing method. Regardless of storage duration, AD and WBD RCC supernatants were immunosuppressive with respect to TNF-α production in response to LPS (Figure 4A). Meanwhile, day 42 WBD supernatants produced more IL-8 in the absence of LPS (Figure 5), suggesting a mixed immunosuppressive and inflammatory response to WBD RCC at day 42. Exposure to day 5 WBF RCC supernatant resulted in increased LPS-induced IL-1β production (Figure 4B) and higher IL-8 in the absence of LPS (Figure 5), suggesting an augmented inflammatory response to fresh WBF RCC (Figure 4C). Monocyte LPS-induced IL-10 and IL-8 production did not differ from controls for any of the RCC supernatants evaluated (Figure 4B,D).
Monocyte LPS-induced cytokine production. (A) TNF-α, (B) IL-10, (C) IL-1β, and (D) IL-8 following exposure of RCC supernatant from different RCC manufacturing methods at fresh (day 5/white) and at expiration (day 42/shaded). Significant level in comparison with control (*P < .05; **P < .01; ***P < .001).
Monocyte LPS-induced cytokine production. (A) TNF-α, (B) IL-10, (C) IL-1β, and (D) IL-8 following exposure of RCC supernatant from different RCC manufacturing methods at fresh (day 5/white) and at expiration (day 42/shaded). Significant level in comparison with control (*P < .05; **P < .01; ***P < .001).
Monocyte IL-8 production in the absence of LPS stimulation and following exposure to RCC supernatant from different RCC manufacturing methods at fresh (day 5/white) and at expiration (day 42/shaded). *Significant level in comparison with control (***P < .001; ****P < .0001).
Monocyte IL-8 production in the absence of LPS stimulation and following exposure to RCC supernatant from different RCC manufacturing methods at fresh (day 5/white) and at expiration (day 42/shaded). *Significant level in comparison with control (***P < .001; ****P < .0001).
Correlations among residual cells, EV, and cytokine production
Exploratory correlational analyses were performed to assess the relationships between monocyte function and the amount of residual cells with all of the RCC products (supplemental Figure 1). Significant and clear negative correlations were identified between residual platelet count and LPS-induced pro-inflammatory cytokine production: TNF-α (r = 0.543, P = .002) and LPS IL-8 (r = 0.507, P = .005), suggesting that higher residual platelet counts are associated with immunosuppressive activity (supplemental Figure 1A,C). Similarly, residual platelet count was negatively correlated with IL-8 production in the absence of LPS, again suggesting a potentially anti-inflammatory phenotype (r = 0.550, P = .003) (supplemental Figure 1D). Conversely, there were no strong correlations identified between residual WBC and monocyte cytokine production, although the correlation between residual WBC and LPS-induced IL-10 was statistically significant (P = .043, r = 0.378) (supplemental Figure 1E-H).
Additional correlation analyses were executed to evaluate the relationships between the monocyte function and cell-derived EVs. For fresh RCC products, no significant correlation was found between cytokine production and platelet-EV, RBC-EV, or total WBC-EV (supplemental Table 1a). However, as presented in supplemental Table 1a, significant moderate negative correlations were identified between LPS-induced TNF-α and B cell–derived and monocyte-derived-EV (CD19+EV [0.437, P = .017] and CD16+EV [0.467, P = .010)] in fresh products. Likewise, LPS-induced IL-10 significantly and negatively correlated with B cell–derived, monocyte-derived, and T cell–derived EV (CD19+EV [r = 0.513, P = .004], CD16+EV [r = 0.499, P = .005], and CD3+EV [r = 0.379, P = .042]).
At day 42 of storage, there was a significant negative correlation between platelet-EV and LPS-induced TNF-α (r = 0.352, P = .048; supplemental Table 1b). In the absence of LPS stimulation, a clear positive correlation was identified between IL-8 and total WBC-EV as well as CD14+ monocyte-EV (r = 0.570, P = .001; r = 0.610, P = .0004, respectively; supplemental Table 1b).
Discussion
In this study, different manufacturing methods influenced the quality control parameters and EV characteristics of RCC products and were associated with differential immunomodulatory activity in vitro. Our findings are in agreement with previously published studies documenting differences in RCC quality measures, including levels of hemolysis, potassium, deformability, and residual plasma, platelet and leukocyte concentrations, and EV quantities across manufacturing method.30,32,41,42 It is no longer appropriate to consider all RCC used in transfusion as equivalent. The current study is among the first to document a potential functional consequence related to these differences.
Although factors associated with TRIM are yet to be fully elucidated, studies have suggested that the infusion of damaged or active cells, and/or foreign antigens/mediators in both soluble and cell-associated forms, are potential immunomodulatory mediators that are strongly associated with TRIM.7,12,13,43 Several studies have shown that RCC products contain residual cells and accumulate cell-derived factors in the supernatant during storage, such as EVs, which have been shown to have proinflammatory and immunosuppressive potential.30,44-47 For instance, in a publication by Danesh et al in 2014,47 the authors demonstrated proinflammatory effects, including increased release of proinflammatory cytokines from monocytes after incubation with exosomes (small EV) isolated from RCC, suggesting that RCC may contribute to TRIM. Conversely, in other previous work by our group, supernatants from leukoreduced stored RCC that had been depleted of EVs suppress monocyte function in vitro and extracellular protein-bound RNA, such as microRNA, were implicated as a potential soluble mediator of immunosuppression.40
In this study, an immunosuppressive effect was identified with AD and WBD RCC supernatants as shown by the significant reduction in the release of the inflammatory cytokine (TNF-α) by monocytes in response to LPS stimulation. TNF-α is an important cytokine in immune activation and antimicrobial immunity.48-50 In clinical studies, low whole blood TNF-α production in response to LPS is a reproducible marker of immune suppression in critically ill patients, associated with risks of nosocomial infection, prolonged organ dysfunction, and death.51-53 Our findings are in agreement with previous studies reporting similar immunosuppressive activity of WBD RCC products.39,54
In our exploratory analyses relating immunomodulatory activity to cell-derived EV, a statistically significant correlation was identified in this study between platelet-derived EV and the suppressed LPS-induced TNF-α production in RCC at expiry. Similar to what was observed for residual platelets, there was a negative correlation between platelet-EV and LPS-induced TNF-α production, suggesting that platelet-derived EVs correlated with immunosuppressive activity. Because neither residual cells nor EV population correlations perfectly explain the mixed immunomodulatory effects observed with different blood manufacturing methods, it is likely that other mediator(s) in the supernatant of the blood products might play an important role in these effects. Although the focus of this study was not to analyze the soluble immunomodulator factors in the blood product supernatant, the immunomodulatory roles of several soluble mediators, including platelet-derived mediators, have been examined. For instance, Perros et al55 showed that supernatant from platelet concentrate cocultured with dendritic cells resulted in significant immunosuppression as evidenced by downregulated IL-12, IL-6, IL-1α, and TNF-α. It has been indicated that this could be due to soluble mediators present in the supernatant such as histamine, platelet factor 4, and sCD40L, which can regulate the expression and the production of cytokines and chemokines.55 Furthermore, Ando et al56 revealed that platelets upon stimulation secrete suppressive soluble factors, more likely to be protein(s), which may downregulate the macrophage responses without direct cell–cell contact. Recent work from our group showed that platelet-EVs induced TGF-β secretion without inducing proinflammatory cytokines in EV-exposed monocytes.57
Interestingly, our study failed to identify significant correlations between RBC-EV and monocyte cytokine production across manufacture methods for either fresh RCC or RCC at expiration, consistent with our recent publication measuring effects of RBC-EV on monocyte activation.57 Previous studies suggest an immunosuppressive role of RBC-EV; Sadallah et al58 observed a significant reduction in the release of LPS-induced inflammatory cytokines (TNF-α, IL-8) in the presence of exosomes derived from isolated erythrocytes. They postulated that the immunosuppressive effects could be due to phosphatidylserine expressed on the surface of the RBC-EV, which have been shown to downregulate the immune response. It has been also suggested that the RBC-EV react with Toll-like receptors and downregulate their ability to activate the macrophage in the presence of LPS stimulation.58 Whether transfusion of these EV within the RCC product may account for some of reported immunosuppressive activity associated with transfusion remains uncertain and requires further investigations.
Although an immunosuppressive effect was observed with the supernatant from AD and WBD RCC, supernatants from fresh WBF units resulted in significantly higher inflammatory cytokine (IL-8) production from the unstimulated monocyte model in comparison with controls. IL-8 is a very important mediator and regulator of the innate immune response.59 It is also believed to be a valuable diagnostic tool because it has been used along with other cytokines, such as IL-6, to determine the severity of inflammation in the body before death.59,60 Interestingly, fresh WBF units, which were associated with higher IL-8 production in the absence of LPS, were shown to have lower residual platelets. At the same time, RCC supernatants that resulted in monocyte IL-8 expression similar to control values had higher residual platelet counts, suggesting that perhaps residual platelets may blunt inflammatory effects of other mediators in this model. Therefore, the effect of residual platelets on immunomodulatory activity and patient clinical outcomes is worth additional examination.
The augmented inflammatory responses associated with “fresh” but not “expired” WBF products is a novel finding that could provide a biological mechanism for the data recently published by Heddle et al.20 In that registry study, transfusion of fresh (≤7 days of storage) WBF was associated with higher in-hospital mortality compared with the mid-age (8 to 35 days) of the reference group (RCF RCCs). Collectively, our work suggests that storage duration and blood manufacturing method used to produce the blood components could both affect patient clinical outcomes. However, additional investigation is warranted to validate and explain these findings, and to identify the causative factors associated with these outcomes.
Our study has limitations. It focused on the cytokine production of monocytes because of its clinical relevance, especially in critically ill patients. However, the immunomodulatory effects of RCC supernatant on other immune cell types or on other measures of the monocyte function may be different. Similarly, in vitro models may not reflect the complexity of the biological system in vivo and the interactions between immune and nonimmune cells, endothelial cells, and microenvironment, which all may influence host response to transfusion. Furthermore, in this study, we examined only fresh (day 5) and expired (day 42) RCC supernatant because it covers the storage time range for RCC transfusion, but earlier points such as day 0 or day 1 may better reflect the influence of manufacturing methods without a storage effect. In addition, we did not measure the effect of the EV-free supernatant or the potential soluble immunomediators in the supernatant; it is likely that these factors may play an important role in the mixed immunomodulatory effects observed with different blood manufacturing methods. We view these as important future studies. Furthermore, we centrifuged the blood product to collect the supernatant for testing, and it is possible that the centrifugation may generate more EV in the supernatant and may release the cargo of some cells or particles, which may affect the final results of this study. Moreover, not all EV in this study were categorized based on their cell of origin given the small EVs/exosomes that were detected by the TRPS technique but were not identified by the flow cytometer. Thus, the exploratory correlation analysis relating immunomodulatory activity to cell-derived EVs did not include all EVs, but rather those large enough to be detected on the flow cytometer (>100-150 nm). Furthermore, the correlation analysis performed here was an exploratory correlation only; we did not correct for multiple comparisons in the correlations because of hypothesis-generating exploratory data. Additionally, it is not yet clear whether these findings and differences observed are due to the differences in manufacturing methods or to other variables such as donor characteristics. Although donor factors such as sex and age may influence RCC products during storage,61 the main focus of this project was to investigate the effect of different manufacturing methods on RCC characteristics and immunomodulatory effects on monocyte activity.
In conclusion, this study shows that blood manufacturing methods significantly influence the immunomodulatory effects of RCC supernatant on monocytes in vitro and significantly affect RBC and non-RBC EV characteristics throughout storage, which have the potential to affect quality and safety of RBC products. Effects were largely independent of storage duration, suggesting that the differences observed between RCC manufacturing methods may account for differences in studies examining clinical effects of RCCs storage duration, particularly within international multicenter studies. Results warrant further examination of their potential immunomodulatory effects and clinical consequences.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Anita Howell, Tracey Turner, Angela Hill, April Xu (Centre for Innovation, Canadian Blood Services), and Luciana da Silveira Cavalcante (Department of Laboratory Medicine and Pathology, University of Alberta) for their technical support. The generous donation of all of our blood donors is gratefully acknowledged. The authors also acknowledge Qi-long Yi, Canadian Blood Services statistician, for assistance with data analysis.
This work was funded by grants from the Canadian Blood Services Intramural Grant program (2015IG-JA); Canadian Blood Services, which is funded by the Federal (Health Canada), Provincial, and Territorial Ministries of Health; and the National Institutes of Health, National Heart, Lung, and Blood Institute (grant K08HL123925) (J.A.M.). R.J.A. is supported by the Saudi Arabian Cultural Bureau in Canada as a recipient of a scholarship from the Government of Saudi Arabia.
The views expressed herein do not represent the views of the Canadian federal government.
Authorship
Contribution: R.J.A., H.I., and S.M. performed the experiments; P.J.N., S.P., and J.P.A. sourced the products used in this study; R.J.A., P.J.N., M.R.W., P.C.S., J.A.M., N.J., and J.P.A. analyzed the results; R.J.A. and J.A.M. prepared the figures; and R.J.A., P.J.N., N.J., P.C.S., J.P.A., and J.A.M. designed the research and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason P. Acker, Canadian Blood Services, 8249 114 St, Edmonton, AB T6G 2R8, Canada; e-mail: jason.acker@blood.ca.