Key Points
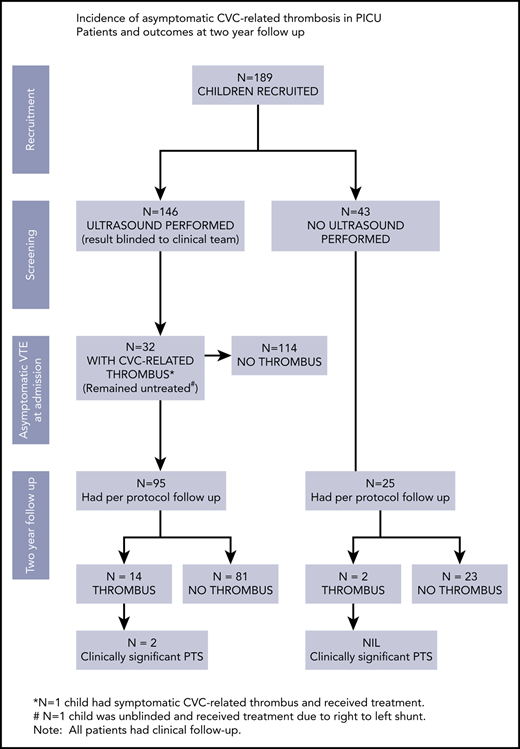
Among 189 children, 31 children had asymptomatic CVC-related thrombus but despite no treatment, only 1 child had mild long-term sequelae.
Urgent femoral CVC placement predicted residual thrombosis at 2 years (P = .02) and was associated with increased acuity and complication.
Abstract
Asymptomatic central venous catheter (CVC)–related thrombosis in children varies in incidence from 5% to 69%. The rate of acute and long-term complications, such as postthrombotic syndrome (PTS), from asymptomatic CVC-related thrombosis is unknown. This article reports the outcomes of a prospective study of 189 children in pediatric intensive care that aimed to determine the frequency of asymptomatic CVC-related thrombosis during hospital admission, and the incidence of residual CVC-related thrombosis and clinically significant PTS 2 years later. Risk factors associated with CVC-related thrombosis were also identified. This study is distinct from previous work as children identified to have asymptomatic CVC-related thrombosis were not treated (clinical team kept blinded) and the entire cohort was followed for 2 years to determine the natural history of asymptomatic thrombosis. Ultrasounds of 146 children determined a 21.9% incidence of acute CVC-related thrombosis. Two children were symptomatic. No radiological thrombosis extension or clinical embolization occurred in the 126 children assessed at follow-up. Using 2 recognized PTS scales, clinically significant PTS was reported in 2 children (1 symptomatic, 1 asymptomatic CVC-related thrombosis), however, neither had functional impairment. Cardiac arrest was a risk factor for CVC-related thrombosis during admission and femoral CVC placement was predictive of residual thrombosis 2 years later. This study challenges the notion that critically ill children with asymptomatic CVC-related thrombosis require anticoagulant treatment, as the results demonstrate that the incidence of acute or long-term complications is low. A larger confirmatory study of nontreatment of CVC-related thrombosis in critically ill children is justified.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 884.
Disclosures
Associate Editor Thomas L. Ortel, CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Determine the incidence of central venous catheter-related thrombosis (CVC-RT) in a pediatric population and associated risk factors, based on a prospective cohort study
Assess mortality, long-term complications, and postthrombotic syndrome after CVC-RT in a pediatric population, based on a prospective cohort study
Evaluate the clinical implications of these findings regarding CVC-RT in a pediatric population, based on a prospective cohort study
Release date: February 21, 2019; Expiration date: February 21, 2020
Introduction
The association between central venous catheters (CVCs) and venous thrombosis in children is well known. Acute complications of CVC-related thrombosis (CVC-RT) include catheter occlusion, pain, loss of venous access, pulmonary embolism, and paradoxical stroke. Long-term, CVC-RT can lead to postthrombotic syndrome (PTS). The true incidence of CVC-RT in children remains unclear as screening for asymptomatic CVC-RT in unselected cohorts of children has been conducted in small studies with large variation in the incidence. Hence, the clinical outcomes, short- and long-term, of asymptomatic CVC-RT are unknown.
Six studies investigated CVC-RT in neonates and children with congenital heart disease (CHD), reporting rates from 0.8% to 43.7%.1-6 Only 4 of these studies screened for asymptomatic CVC-RT. In 2 older prospective studies conducted in North American pediatric intensive care units (PICUs), the incidence of asymptomatic CVC-RT was 13.1% in a sample of 76 children and 35% in 20 children with femoral CVCs.7,8 Most recently, a study by Faustino et al reported an incidence of asymptomatic CVC-RT of 15.8% in a cohort of 101 children in PICU.9
Risk factors for CVC-RT identified in critically ill children include elevated factor VIII, increasing age, blood transfusion, and the site of CVC placement.10-12 However, these factors have only been reported in single studies with no validation in other cohorts. A meta-analysis of 16 studies of thrombophilia in children with CVC-RT reported that the presence of 1 or more thrombophilia traits (inherited or acquired) was associated with the development of CVC-RT.12 The association between thrombophilia was stronger when measured in children with symptomatic CVC-RT compared with children with asymptomatic CVC-RT.12
The current American College of Chest Physicians guidelines recommend treatment of CVC-RT with anticoagulation with low-molecular-weight heparin (LMWH) for 6 to 12 weeks (grade 2C).13 This therapy conveys significant risks, such as major bleeding, especially in critically ill children. The decision to treat CVC-RT with anticoagulation is often dependent on the presence of other thrombotic risk factors, such as CHD.5,14-16 Decisions about treatment of CVC-RT are also influenced by the perceived risk of long-term sequelae, specifically PTS. Yet, the risk of PTS from untreated asymptomatic CVC-RT is unknown and the long-term thrombotic burden from asymptomatic CVC-RT has not been investigated.
This article reports the outcomes of a prospective study of 189 children in PICUs that aimed to determine the frequency of asymptomatic CVC-RT during hospital admission, and the incidence of residual CVC-RT and clinically significant PTS 2 years later. The study also sought to identify risk factors associated with CVC-RT and clinically significant PTS. This study is distinct from previous work as children in this cohort identified to have asymptomatic CVC-RT were not treated (clinical team blinded to imaging results). The cohort was followed for 2 years to determine the natural history of asymptomatic CVC-RT.
Methods
This prospective cohort study recruited children admitted to PICUs requiring a CVC in the jugular or femoral veins that remained in situ for more than 24 hours. Children were ineligible for the study if they had had a CVC placed into the subclavian vein, if they had a previous CVC within the same blood vessel within the last 3 years, or if they had no planned follow-up at the institution. Children with a CVC placed into the subclavian vein were excluded as imaging of this vessel via ultrasound is insensitive to the detection of thrombus as compressibility of veins cannot be assessed within the thoracic cage and ultrasound was the only imaging modality available.17
Ethics approval was obtained from the hospital human research ethics committee (#62063) and informed consent was obtained from a parent/guardian of each child. Patients and their parents were approached about the study during cardiac surgery preadmission clinic (all children requiring elective cardiac surgery have a CVC placed), on PICU or on the cardiac ward.
Data collection
Admission
Participants had an ultrasound on the side where the CVC was placed during their admission. The results of the ultrasound were blinded to the clinical team. If a child developed clinical signs or symptoms of a CVC-RT (swelling, pain, edema, redness at the site of CVC insertion, and CVC occlusion) within 24 hours of the study ultrasound then the result of the ultrasound was unblinded. If outside of 24 hours, then a separate ultrasound was performed as per standard practice.
Ultrasounds were performed within 72 hours of the CVC insertion, or, if this was not feasible, then ultrasounds were performed prior to the child’s hospital discharge. All ultrasound images were acquired using the Siemens S2000 ultrasound machine. When the CVC was placed into the jugular vein, ultrasound was performed over the jugular, brachiocephalic, subclavian vein on the same side as the CVC. The contralateral side was not examined. If the CVC was placed into the right or left femoral vein, the ultrasound examined the external iliac vein, common femoral vein and femoral vein ipsilateral to the CVC. Three senior sonographers conducted all ultrasounds and 2 consultant radiologists were responsible for reporting all study ultrasounds. Interrater reliability of reporting was assessed.
Filling defects were used to identify thrombosis and were characterized as occlusive thrombus, nonocclusive thrombus, or fibrin bands/sheaths. Wall thickening was also reported. A priori definitions of thrombosis are presented in Table 1.
Study definitions
| Outcome . | Definition . |
|---|---|
| Asymptomatic CVC-related thrombosis | The presence of occlusive or nonocclusive thrombus in at least 1 vessel as identified on Doppler ultrasonography and the absence of any clinical signs or symptoms of thrombosis of the area. |
| Symptomatic CVC-related thrombosis | The presence of occlusive or nonocclusive thrombus in at least 1 vessel as identified on Doppler ultrasonography and the presence of at least 1 of the following signs or symptoms: swelling, pain, redness, or discoloration of the area; dysfunction of the CVC. |
| Occlusive thrombosis | Any echogenic filling defect that completely occludes flow through a named vessel and/ or inability to compress the vessel. |
| Nonocclusive thrombosis | A echogenic filling defect that partially occluded flow through 1 named vessel; fibrin sheath/ band causing luminal narrowing; catheter tip thrombus. |
| Wall thickening | Excessive thickening or calcification of a vessel wall. |
| Extensive CVC-related thrombosis | Occlusive thrombus in 1 or more vessel; nonocclusive thrombus in >1 vessel with or without wall thickening. |
| Clinically significant PTS | MJI: clinically significant PTS is defined as the presence of both a physical sign and a functional impairment (ie, a score of at least 1 in both the physical and functional categories).30-32 |
| MV scale: Moderate PTS is defined as a score of 4 to 8; a score of >8 is regarded as severe PTS. | |
| Moderate or Severe PTS as defined by the MVS is classified as clinically significant PTS in this study. | |
| PIM2 score | Calculated within an hour of admission to PICU. Collected as a probability of death score.18,33 |
| Major bleeding | “Composite of” fatal bleeding; clinically overt bleeding associated with a fall in hemoglobin level of 20 g L or more in a 24-h period; bleeding that is retroperitoneal, pulmonary, intracranial or involves the central nervous system; and bleeding that requires surgical intervention in an operating suite34 (p1857). |
| Clinically relevant nonmajor bleeding | A composite of overt bleeding requiring a blood transfusion which “is not directly attributable to the patient’s underlying medical condition and bleeding that requires medical or surgical intervention to restore haemostasis, other than in an operating suite”34 (p1857). |
| Outcome . | Definition . |
|---|---|
| Asymptomatic CVC-related thrombosis | The presence of occlusive or nonocclusive thrombus in at least 1 vessel as identified on Doppler ultrasonography and the absence of any clinical signs or symptoms of thrombosis of the area. |
| Symptomatic CVC-related thrombosis | The presence of occlusive or nonocclusive thrombus in at least 1 vessel as identified on Doppler ultrasonography and the presence of at least 1 of the following signs or symptoms: swelling, pain, redness, or discoloration of the area; dysfunction of the CVC. |
| Occlusive thrombosis | Any echogenic filling defect that completely occludes flow through a named vessel and/ or inability to compress the vessel. |
| Nonocclusive thrombosis | A echogenic filling defect that partially occluded flow through 1 named vessel; fibrin sheath/ band causing luminal narrowing; catheter tip thrombus. |
| Wall thickening | Excessive thickening or calcification of a vessel wall. |
| Extensive CVC-related thrombosis | Occlusive thrombus in 1 or more vessel; nonocclusive thrombus in >1 vessel with or without wall thickening. |
| Clinically significant PTS | MJI: clinically significant PTS is defined as the presence of both a physical sign and a functional impairment (ie, a score of at least 1 in both the physical and functional categories).30-32 |
| MV scale: Moderate PTS is defined as a score of 4 to 8; a score of >8 is regarded as severe PTS. | |
| Moderate or Severe PTS as defined by the MVS is classified as clinically significant PTS in this study. | |
| PIM2 score | Calculated within an hour of admission to PICU. Collected as a probability of death score.18,33 |
| Major bleeding | “Composite of” fatal bleeding; clinically overt bleeding associated with a fall in hemoglobin level of 20 g L or more in a 24-h period; bleeding that is retroperitoneal, pulmonary, intracranial or involves the central nervous system; and bleeding that requires surgical intervention in an operating suite34 (p1857). |
| Clinically relevant nonmajor bleeding | A composite of overt bleeding requiring a blood transfusion which “is not directly attributable to the patient’s underlying medical condition and bleeding that requires medical or surgical intervention to restore haemostasis, other than in an operating suite”34 (p1857). |
Study radiologists could unblind ultrasound results at their own discretion, if they felt the child was at high risk of thromboembolism due to extensive thrombus size and mobility. The first author (S.J.) remained blinded to all admission and follow-up ultrasound reports until after the PTS assessments were performed at follow-up.
Demographic and clinical data and plasma were collected during the child’s admission to determine risk factors for CVC-RT and PTS. Incidences of major and clinically relevant bleeding as defined in Table 1 were recorded and severity of illness was measured using the Pediatric Index of Mortality 2 (PIM2) score.18 The study used residual plasma from blood collected from the children for routine clinically indicated coagulation tests during their admission to test D-dimer and factor VIII assays. If there was no residual plasma available from coagulation tests, no additional blood was taken for study purposes. D-dimer and factor VIII assays were performed using a STA-Liatest D-Di Plus (Diagnostica Stago, Asnières, France) kit and analyzed using the STAR-Evolution analyzer (Diagnostica Stago). The results are expressed in nanograms per milliliter (as fibrinogen equivalent units) for D-dimer and factor VIII activity is expressed as a percentage.
Two-year follow-up
An ultrasound and a PTS assessment using 2 pediatric PTS tools, the Manco-Johnson Instrument (MJI) and the Modified Villalta (MV), were performed ∼24 months following CVC placement.19,20 The ultrasound was performed as per the procedure described for the admission ultrasound. The definitions of PTS and clinically significant PTS using the MJI and MV are presented in Table 1.
Statistical analysis
All data analysis was performed using Statistical Package for the Social Sciences (SPSS) (version 20.0.0, 2011; IBM Corporation). Descriptive statistics were performed to analyze all outcome variables and define the demographic characteristics of the sample.
The factors suspected to be associated with the development of CVC-RT were analyzed independently. Continuous variables that were normally distributed were compared using the Student t test and continuous variables that were skewed were compared using the Mann Whitney U test. Categorical variables were compared using the χ2 test. Odds ratios (ORs) and relative risks with 95% confidence intervals (CIs) were calculated using standard methods, comparing participants with and without CVC-RT at admission and follow-up, and participants with and without clinically significant PTS. Statistical significance was set at P ≤ .05.
Interrater reliability was measured by percentage agreement, calculated as the number of agreeing observations divided by the total number of observations.
Results
Demographics
Over 16 months, 205 children with a jugular or femoral CVC were consented for this study. Sixteen children became ineligible due to no CVC being placed (n = 5, 2.6%) (in patients consented at cardiac surgery preadmission clinic); the CVC did not remain in situ for 24 hours (n = 6, 3.2%); the CVC was placed into the same vessel as a previous CVC (n = 4, 2.1%) and 1 patient was deemed ineligible as consent was given by the child’s foster parents but could not be obtained from the child’s legal guardians.
As presented in Table 2, over 75% of the cohort had an underlying diagnosis of CHD and nearly 69% of children required admission to PICU following cardiac surgery. The median age for the sample was 12 months and 23% of the cohort were neonates. CVCs were in situ for a median time of 4 days (range, 1-36 days). The most common complication experienced by children in this cohort was an episode of hypotension (29.1%) and 12.7% of children had a cardiac arrest during their hospital admission. The majority of patients (83.1%) had an unfractionated heparin (UFH) infusion commenced within 12 hours of CVC insertion and the mean dose of UFH being administered continuously was 10.4 units per kilogram per hour (standard deviation, 3.8 units per kg/h).
Cohort demographics
| . | Number of patients . | Incidence, % . |
|---|---|---|
| Eligible consented patients | ||
| Age of patients at CVC insertion, median (range), y | 1 (0-17) | |
| Male sex | 98 | 51.9 |
| Weight, median (range), kg | 8.7 (2.2-90) | |
| Primary diagnosis | ||
| Cyanotic CHD | 42 | 22.2 |
| Acyanotic CHD | 100 | 52.9 |
| Other cardiac | 8 | 34.2 |
| Respiratory | 9 | 4.8 |
| Sepsis/viral illness | 8 | 4.2 |
| Neurology/neurosurgery | 8 | 4.2 |
| Orthopedic surgery | 6 | 3.2 |
| Motor vehicle accident | 3 | 1.6 |
| Renal failure | 3 | 1.6 |
| Burns | 1 | 0.55 |
| Liver transplant | 1 | 0.55 |
| CVC characteristics | ||
| Time in situ, median (range), d | 4 (1-36) | |
| Jugular | 154 | 81.5 |
| Femoral | 35 | 18.5 |
| Unit inserted, n (%) | ||
| Operating theater | 144 (76.2) | |
| PICU | 42 (22.2) | |
| Other* | 3 (1.6) | |
| Parameter | ||
| Dose UFH thromboprophylaxis, mean (SD), units per kg/h | 157 | 10.4 (3.8) |
| PIM2 score, median (range) | 180 | 2.0 (0.14-100) |
| D-dimer, median (range) | 184 | 0.71 (0.27-20) |
| Factor VIII, median (range) | 56 | 178 (59-516) |
| . | Number of patients . | Incidence, % . |
|---|---|---|
| Eligible consented patients | ||
| Age of patients at CVC insertion, median (range), y | 1 (0-17) | |
| Male sex | 98 | 51.9 |
| Weight, median (range), kg | 8.7 (2.2-90) | |
| Primary diagnosis | ||
| Cyanotic CHD | 42 | 22.2 |
| Acyanotic CHD | 100 | 52.9 |
| Other cardiac | 8 | 34.2 |
| Respiratory | 9 | 4.8 |
| Sepsis/viral illness | 8 | 4.2 |
| Neurology/neurosurgery | 8 | 4.2 |
| Orthopedic surgery | 6 | 3.2 |
| Motor vehicle accident | 3 | 1.6 |
| Renal failure | 3 | 1.6 |
| Burns | 1 | 0.55 |
| Liver transplant | 1 | 0.55 |
| CVC characteristics | ||
| Time in situ, median (range), d | 4 (1-36) | |
| Jugular | 154 | 81.5 |
| Femoral | 35 | 18.5 |
| Unit inserted, n (%) | ||
| Operating theater | 144 (76.2) | |
| PICU | 42 (22.2) | |
| Other* | 3 (1.6) | |
| Parameter | ||
| Dose UFH thromboprophylaxis, mean (SD), units per kg/h | 157 | 10.4 (3.8) |
| PIM2 score, median (range) | 180 | 2.0 (0.14-100) |
| D-dimer, median (range) | 184 | 0.71 (0.27-20) |
| Factor VIII, median (range) | 56 | 178 (59-516) |
From total sample of n = 189.
SD, standard deviation; UFH, unfractionated heparin.
Angiography suite, Pediatric Emergency Transport Service, Emergency Department.
Incidence of CVC-RT: admission and follow-up
One hundred and forty-six patients had an ultrasound performed during their admission (Table 3). Ultrasounds were not performed for 43 children because the dressing obstructed the view of the vessels (n = 4), the parents refused (n = 2) or the ultrasound could not be performed prior to the patients’ discharge (n = 37). There was a statistically significant difference in the median days the CVC was in situ between children who had an ultrasound performed and those who did not (5 days vs 2 days, respectively; P < .001). However, there were no significant differences in the demographic characteristics of patients who did and did not have an ultrasound performed.
Ultrasound results at admission and 2-year follow-up
| Ultrasound result . | Total, n (%) . | Jugular, n (%) . | Femoral, n (%) . |
|---|---|---|---|
| Admission | |||
| Total | 146 | 113 | 33 |
| Normal | 100 (68.5) | 84 (74.4) | 16 (48.5) |
| Thrombus | 32 (21.9)* | 21 (18.6) | 11 (33.3) |
| Wall thickening | 14 (9.6) | 8 (7) | 6 (18.2) |
| 2-year follow-up (with imaging at admission), n = 95 | |||
| Normal | 65 (68.4) | 59 (84.3) | 6 (24) |
| Thrombus | 14 (14.7) | 6 (8.6) | 8 (32) |
| Wall thickening | 16 (16.9) | 5 (7.1) | 11 (44) |
| 2-year follow-up (no imaging at admission), n = 25 | |||
| Normal | 21 | 21 (84) | 0 |
| Thrombus | 2 | 2 (8) | 0 |
| Wall thickening | 2 | 2 (8) | 0 |
| Total no. followed up | 120 | 95 | 25 |
| Ultrasound result . | Total, n (%) . | Jugular, n (%) . | Femoral, n (%) . |
|---|---|---|---|
| Admission | |||
| Total | 146 | 113 | 33 |
| Normal | 100 (68.5) | 84 (74.4) | 16 (48.5) |
| Thrombus | 32 (21.9)* | 21 (18.6) | 11 (33.3) |
| Wall thickening | 14 (9.6) | 8 (7) | 6 (18.2) |
| 2-year follow-up (with imaging at admission), n = 95 | |||
| Normal | 65 (68.4) | 59 (84.3) | 6 (24) |
| Thrombus | 14 (14.7) | 6 (8.6) | 8 (32) |
| Wall thickening | 16 (16.9) | 5 (7.1) | 11 (44) |
| 2-year follow-up (no imaging at admission), n = 25 | |||
| Normal | 21 | 21 (84) | 0 |
| Thrombus | 2 | 2 (8) | 0 |
| Wall thickening | 2 | 2 (8) | 0 |
| Total no. followed up | 120 | 95 | 25 |
n = 1 patient had symptomatic CVC-related thrombosis.
CVC-RT (both symptomatic and asymptomatic) was found in 32 of 146 imaged patients (21.9%). One patient had symptomatic CVC-RT at the time of their first ultrasound. Another patient developed symptomatic CVC-RT 4 days after a negative study ultrasound. Both patients were receiving UFH prophylaxis at doses of 10 units per kg/h and 15 units per kg/h respectively.
Eight children were identified to have extensive CVC-RT as defined in Table 1. Of these, 2 patients had occlusive thrombus in the external iliac vein; 1 patient was symptomatic and received standard therapeutic anticoagulation treatment; the other patient also had nonocclusive thrombus in the common femoral vein but was asymptomatic.
The clinical team was informed about 1 child with asymptomatic CVC-RT in the brachiocephalic vein as the radiologist was concerned about a large and potentially mobile thrombus (occluded 80% of the vein). The child had an unrepaired ventricular septal defect and was felt to be at risk of paradoxical thromboembolism due to the presence of a right to left shunt. The clinical team made the decision to treat the child with 6 weeks of LMWH.
Interrater reliability was calculated using a random sample of 34 patient’s ultrasound reports; 95% agreement between the 2 radiologists was achieved.
Mortality
Prior to follow-up, 14 children died (7.4%). Four of the 14 children who died had asymptomatic CVC-RT identified on their admission ultrasound. These 4 children had their medical histories reviewed by 3 independent clinicians; an intensivist, a cardiac surgeon and a hematologist to objectively determine if any of these deaths were related to the thrombus identified on the blinded ultrasound. The 3 clinicians independently and unanimously found that none of the 4 children with asymptomatic thrombus identified on the admission ultrasound died of thromboembolic or hemorrhagic complications. None of the other 10 children who had negative ultrasounds for thrombosis, and died during the study, died of thromboembolic or hemorrhagic complications.
Two-year follow-up
Of the 175 children eligible for follow-up, 73% (n = 128) received study protocolled review. Eight children were assessed for PTS but did not have an ultrasound performed at follow-up due to the child being too distressed (n = 3), missing their scheduled ultrasound appointment (n = 2), or declining attendance for the ultrasound (n = 3). Some follow-up (including routine clinical review) was successfully obtained on all but 1 of the other 47 children. The time to follow-up was a mean of 26 months (standard deviation, 5.2 months) from the time of CVC insertion.
Of the 120 children who had an ultrasound performed at follow-up, 16 children were identified to have thrombus (13.3%) (Table 3). Of the 16 children with thrombus, 3 patients had CVC-RT on the admission ultrasound, 2 patients had wall thickening and 9 patients had no evidence of thrombus on their admission ultrasound. One of these 9 patients was the child that developed symptomatic CVC-RT 4 days after a negative screening ultrasound and received anticoagulation treatment. Two patients with thrombus at follow-up did not have an ultrasound during admission.
No radiological thrombosis extension or clinical embolization (including paradoxical emboli) associated with the CVC-RT occurred in the 128 children assessed at follow-up. One patient admitted post fenestrated-Fontan, had an embolic stroke 22 days after the study CVC was removed. The cause of the stroke was identified to be 2 thrombi within the right atrium that were documented to have developed due to turbulent flow in the right atrium. The child received additional imaging of his jugular veins and there was no evidence of thrombosis. The child was receiving 15 units per kg/h of UFH as thromboprophylaxis at the time of the stroke.
Risk factors for CVC-RT
The number of CVC days, age, D-dimer, factor VIII, and PIM2 probability of death (PIM2) scores were not associated with acute CVC-RT or residual thrombosis in this cohort (all P values >.05). Factors identified to be significantly associated with the development of CVC-RT on bivariate analysis included the unit where the CVC was inserted (PICU, operating theater, other), if the patient received more than 10 units per kg/h of UFH, if the patient had a cardiac arrest and if the patient had a major or clinically relevant bleeding episode (Table 4, all P values ≤.05). However, only 1 of these factors, cardiac arrest, was found to significantly increase the risk of CVC-RT during admission (OR, 3.3; 95% CI, 1.29-8.55; P = .01). The odds of a patient having thrombus present at follow-up were significantly increased if their CVC was placed into the femoral vein (OR, 26.2; P = .02) or they had an episode of hypotension (OR, 2.85; P = .05) (Table 4). Table 5 presents the analysis of the association between the CVC placement and CVC-RT at admission and follow-up.
Clinical factors and association with CVC-related thrombosis
| Factors . | Admission . | Follow-up . | ||||
|---|---|---|---|---|---|---|
| n . | χ2* . | OR (95%CI) . | n . | χ2* . | OR (95%CI) . | |
| P . | P . | |||||
| ECMO | ||||||
| Yes | 14 | 0.6 | 1.4 (0.41-4.8) | 8 | 0.29 | 2.46 (0.45-13.5) |
| No | 126 | P = 0.6 | 109 | P = .3 | ||
| Open chest | ||||||
| Yes | 14 | 0.23 | 2.0 (0.62-6.5) | 7 | 0.2 | 2.98 (0.52-16.98) |
| No | 125 | P = .2 | 110 | P = .2 | ||
| Cardiac arrest | ||||||
| Yes | 23 | 0.01 | 3.32 (1.29-8.55) | 14 | 0.06 | 3.35 (0.89-12.49) |
| No | 117 | P = .01 | 103 | P = .07 | ||
| Age | ||||||
| <1 y | 79 | 0.8 | 0.89 (0.4-1.97) | 54 | 0.24 | 1.9 (0.64-6.04) |
| >1 y | 67 | P = .7 | 66 | P = .2 | ||
| UFH | ||||||
| ≤10 units | 104 | 0.03 | 2.05 (0.63-6.7) | 86 | 0.45 | 0.93 (0.1-8.2) |
| >10 units | 16 | P = .2 | 10 | P = .9 | ||
| Hypotension | ||||||
| Yes | 47 | 0.11 | 1.9 (0.85-4.28) | 35 | 0.05 | 2.85 (0.98-8.34) |
| No | 99 | P = .1 | 85 | P = .05 | ||
| Bleeding | ||||||
| Yes† | 11 | 0.05 | 3.3 (0.93-11.67) | 8 | 0.3 | 2.3 (0.43-12.7) |
| No | 135 | P = .06 | 112 | P = .3 | ||
| Factors . | Admission . | Follow-up . | ||||
|---|---|---|---|---|---|---|
| n . | χ2* . | OR (95%CI) . | n . | χ2* . | OR (95%CI) . | |
| P . | P . | |||||
| ECMO | ||||||
| Yes | 14 | 0.6 | 1.4 (0.41-4.8) | 8 | 0.29 | 2.46 (0.45-13.5) |
| No | 126 | P = 0.6 | 109 | P = .3 | ||
| Open chest | ||||||
| Yes | 14 | 0.23 | 2.0 (0.62-6.5) | 7 | 0.2 | 2.98 (0.52-16.98) |
| No | 125 | P = .2 | 110 | P = .2 | ||
| Cardiac arrest | ||||||
| Yes | 23 | 0.01 | 3.32 (1.29-8.55) | 14 | 0.06 | 3.35 (0.89-12.49) |
| No | 117 | P = .01 | 103 | P = .07 | ||
| Age | ||||||
| <1 y | 79 | 0.8 | 0.89 (0.4-1.97) | 54 | 0.24 | 1.9 (0.64-6.04) |
| >1 y | 67 | P = .7 | 66 | P = .2 | ||
| UFH | ||||||
| ≤10 units | 104 | 0.03 | 2.05 (0.63-6.7) | 86 | 0.45 | 0.93 (0.1-8.2) |
| >10 units | 16 | P = .2 | 10 | P = .9 | ||
| Hypotension | ||||||
| Yes | 47 | 0.11 | 1.9 (0.85-4.28) | 35 | 0.05 | 2.85 (0.98-8.34) |
| No | 99 | P = .1 | 85 | P = .05 | ||
| Bleeding | ||||||
| Yes† | 11 | 0.05 | 3.3 (0.93-11.67) | 8 | 0.3 | 2.3 (0.43-12.7) |
| No | 135 | P = .06 | 112 | P = .3 | ||
ECMO, extracorporeal membrane oxygenation; NB, not all patients received unfractionated heparin.
Two sided.
Major bleeding and clinically relevant bleeding.
CVC placement and CVC-related thrombosis at admission and follow-up
| . | . | . | . | OR (95% CI) . |
|---|---|---|---|---|
| . | Normal/wall thickening . | Occlusive or nonocclusive thrombus . | P (χ2) . | P . |
| Admission | ||||
| Jugular | 92 | 21 | .72 | 1.27 (0.16-9.9) |
| Femoral | 22 | 11 | P = .8 | |
| Follow-up | ||||
| Jugular | 87 | 8 | .002 | 26.17 (1.5-443.2) |
| Femoral | 17 | 8 | P = .02 |
| . | . | . | . | OR (95% CI) . |
|---|---|---|---|---|
| . | Normal/wall thickening . | Occlusive or nonocclusive thrombus . | P (χ2) . | P . |
| Admission | ||||
| Jugular | 92 | 21 | .72 | 1.27 (0.16-9.9) |
| Femoral | 22 | 11 | P = .8 | |
| Follow-up | ||||
| Jugular | 87 | 8 | .002 | 26.17 (1.5-443.2) |
| Femoral | 17 | 8 | P = .02 |
Jugular and femoral CVC placement were tested for their association with D-dimer, Factor-VIII levels and PIM2. D-dimer and Factor-VIII were demonstrated to have a higher median value in patients with a femoral CVC as compared with patients with a jugular CVC. Children with femoral CVCs were also statistically more likely to have a period of hypotension, a cardiac arrest, have their CVC inserted in PICU and have a non-cardiac diagnosis compared with children with a jugular CVC (Table 6). Children with femoral CVCs were significantly more likely to have a higher PIM2 compared with children with a jugular CVC (OR, 1.041; 95% CI, 1.011-1.072; P = .006), indicating a higher acuity and risk of mortality among children with femoral CVCs. When D-dimer, factor VIII, and PIM2 scores were examined for the sub-group of children with femoral CVCs, there were no differences in values between those with and without CVC-RT at admission or follow-up. However, there was a statistically significant difference in the incidence of major bleeding between children with femoral CVC-RT and children with femoral CVCs (P = .01), but no thrombosis.
Association between CVC placement, laboratory values, and patient characteristics
| . | Jugular CVC . | Femoral CVC . | P . | ||
|---|---|---|---|---|---|
| n . | Median (range), mean (SD), or % of jugular group . | n . | Median (range), mean (SD), or % of femoral group . | ||
| D-dimer | 150 | 0.59 (0.27-10.83) | 35 | 2.35 (0.27-20.0) | <.001* |
| Factor VIII | 48 | 160.5 (59-516) | 8 | 259 (116-399) | .007* |
| CVC time in situ, d | 154 | 5.2 (5.9) | 35 | 9.6 (7.4) | <.001† |
| UFH amount, units | 126 | 10.1 (3.4) | 24 | 12.2 (6.0) | .02† |
| PIM2 score, % | 146 | 1.92 (0.14-100) | 34 | 3.2 (0.30-90.6) | .035* |
| Hypotension | |||||
| Yes | 36 | 23.4 | 19 | 45.7 | <.001‡ |
| No | 118 | 76.6 | 16 | 54.3 | |
| Cardiac arrest | |||||
| Yes | 14 | 9.5 | 10 | 28.6 | .003‡ |
| No | 133 | 90.5 | 25 | 71.4 | |
| ECMO | |||||
| Yes | 9 | 6.1 | 5 | 14.3 | .1‡ |
| No | 138 | 93.9 | 30 | 85.7 | |
| Major bleeding | |||||
| Yes | 11 | 7.1 | 2 | 5.7 | .7‡ |
| No | 143 | 92.9 | 33 | 94.3 | |
| CVC inserted | |||||
| In PICU | 14 | 9.2 | 28 | 82.4 | <.001‡ |
| In OT | 138 | 90.8 | 6 | 17.6 | |
| Diagnosis | |||||
| Cardiac | 139 | 90.3 | 11 | 31.4 | <.001‡ |
| Noncardiac | 15 | 9.7 | 24 | 68.6 | |
| . | Jugular CVC . | Femoral CVC . | P . | ||
|---|---|---|---|---|---|
| n . | Median (range), mean (SD), or % of jugular group . | n . | Median (range), mean (SD), or % of femoral group . | ||
| D-dimer | 150 | 0.59 (0.27-10.83) | 35 | 2.35 (0.27-20.0) | <.001* |
| Factor VIII | 48 | 160.5 (59-516) | 8 | 259 (116-399) | .007* |
| CVC time in situ, d | 154 | 5.2 (5.9) | 35 | 9.6 (7.4) | <.001† |
| UFH amount, units | 126 | 10.1 (3.4) | 24 | 12.2 (6.0) | .02† |
| PIM2 score, % | 146 | 1.92 (0.14-100) | 34 | 3.2 (0.30-90.6) | .035* |
| Hypotension | |||||
| Yes | 36 | 23.4 | 19 | 45.7 | <.001‡ |
| No | 118 | 76.6 | 16 | 54.3 | |
| Cardiac arrest | |||||
| Yes | 14 | 9.5 | 10 | 28.6 | .003‡ |
| No | 133 | 90.5 | 25 | 71.4 | |
| ECMO | |||||
| Yes | 9 | 6.1 | 5 | 14.3 | .1‡ |
| No | 138 | 93.9 | 30 | 85.7 | |
| Major bleeding | |||||
| Yes | 11 | 7.1 | 2 | 5.7 | .7‡ |
| No | 143 | 92.9 | 33 | 94.3 | |
| CVC inserted | |||||
| In PICU | 14 | 9.2 | 28 | 82.4 | <.001‡ |
| In OT | 138 | 90.8 | 6 | 17.6 | |
| Diagnosis | |||||
| Cardiac | 139 | 90.3 | 11 | 31.4 | <.001‡ |
| Noncardiac | 15 | 9.7 | 24 | 68.6 | |
OT, operating theater.
Mann-Whitney U test.
Student t test.
χ2.
Postthrombotic syndrome assessments
PTS assessments were performed for 126 children. The same 13 children were classified as having PTS using the MJI and MV. Two children met the criteria for clinically significant PTS using the MJI, whereas the MV assessed all PTS cases as mild (Table 7). The median age of patients with PTS was 5 years (range, 4 months to 16 years). No child presented with skin changes ascribed to venous disease or ulceration or superior vena cava syndrome. Eight children reported pain, however, only 3 children reported pain in the ipsilateral side to where their CVC was placed. Three children reported pain bilaterally, 1 of whom stated that this was related to swelling of the extremities caused by their underlying autoimmune disease. Two children reported pain bilaterally “all the time” or “after vigorous exercise”; both of whom had complex CHD and thus exercise intolerance was a likely contributor to pain after exercise.
Signs and symptoms and scoring of children with PTS
| CVC placement . | Initial US result . | Follow-up US result . | Abnormal use or pain . | Swelling . | Increased limb circumference* . | Collateral vessels . | Pain present . | MVS final score . | MJI final score . |
|---|---|---|---|---|---|---|---|---|---|
| Jugular right | WT | Not done | Yes† | Yes‡ | No | No | Yes† | 2 | 2 |
| Thrombus | Normal | Yes† | No | No | No | Yes§ | 1 | 1 | |
| Not done | Normal | No | Yes | Yes | No | No | 2 | 1 | |
| Not done | Thrombus | || | Yes† | No | No | || | 1 | 2 | |
| Normal | Normal | No | No | No | Yes | No | 1 | 1 | |
| FS, WT | Normal | No | No | No | Yes | No | 1 | 1 | |
| Thrombus | Normal | Yes | No | No | No | Yes | 1 | 1 | |
| Femoral right | Thrombus, WT | Thrombus | Yes | No | No | No | Yes§ | 1 | 1 |
| Thrombus | Thrombus | No | No | No | Yes | Yes§ | 1 | 2 | |
| Jugular right | Not done | Normal | No | No | No | Yes | Yes† | 1 | 2 |
| Femoral left | WT | WT | No | Yes | Yes | No | No | 2 | 2 |
| Normal¶ | Thrombus | No | Yes | Yes | Yes | Yes | 3 | 3# | |
| Thrombus | WT | Yes | No | Yes | Yes | Yes | 3 | 2# |
| CVC placement . | Initial US result . | Follow-up US result . | Abnormal use or pain . | Swelling . | Increased limb circumference* . | Collateral vessels . | Pain present . | MVS final score . | MJI final score . |
|---|---|---|---|---|---|---|---|---|---|
| Jugular right | WT | Not done | Yes† | Yes‡ | No | No | Yes† | 2 | 2 |
| Thrombus | Normal | Yes† | No | No | No | Yes§ | 1 | 1 | |
| Not done | Normal | No | Yes | Yes | No | No | 2 | 1 | |
| Not done | Thrombus | || | Yes† | No | No | || | 1 | 2 | |
| Normal | Normal | No | No | No | Yes | No | 1 | 1 | |
| FS, WT | Normal | No | No | No | Yes | No | 1 | 1 | |
| Thrombus | Normal | Yes | No | No | No | Yes | 1 | 1 | |
| Femoral right | Thrombus, WT | Thrombus | Yes | No | No | No | Yes§ | 1 | 1 |
| Thrombus | Thrombus | No | No | No | Yes | Yes§ | 1 | 2 | |
| Jugular right | Not done | Normal | No | No | No | Yes | Yes† | 1 | 2 |
| Femoral left | WT | WT | No | Yes | Yes | No | No | 2 | 2 |
| Normal¶ | Thrombus | No | Yes | Yes | Yes | Yes | 3 | 3# | |
| Thrombus | WT | Yes | No | Yes | Yes | Yes | 3 | 2# |
FS, fibrin sheath; US, ultrasound; WT, wall thickening.
Proximal, >1 cm increase in affected extremity compared to contralateral side.
Related to underlying disease, postexercise.
Bilateral and related to underlying disease.
Bilateral pain postaerobic exercise.
Unable to assess pain due to developmental delay or age.
Patient developed symptomatic CVC-related thrombus 4 days postultrasound.
Clinically significant PTS as defined as a score of ≥1 on both the physical and functional scale.
There was no increased risk of clinically significant PTS related to D-dimer levels (P = .2; OR, 1.029 [0.66-1.57]) and PIM2 scores (P = .1). The association between factor VIII levels and clinically significant PTS could not be determined as no children with clinically significant PTS had a Factor-VIII assay performed.
Discussion
We report a prospective study of children admitted to PICU who had a CVC inserted in the jugular or femoral veins. The majority of children (78.5%) who participated in this study required a CVC following cardiac surgery for CHD or to manage their deteriorating cardiac function. The incidence of asymptomatic CVC-RT was 22%, as detected by screening ultrasound, despite routine thromboprophylaxis with UFH. All but 1 child had their ultrasound result blinded to the treating team, and follow-up 2 years post admission demonstrated no significant acute or longer-term sequelae. Our results confirm that UFH thromboprophylaxis may not be effective and furthermore, suggest that asymptomatic CVC-RT may not require therapeutic anticoagulation.
The rate of asymptomatic CVC-RT in the current study was similar to that reported in the KIDCAT study, (21% and 22%, respectively).3 In both studies the majority of children had an underlying diagnosis of CHD and had their CVC placed into the jugular veins (97% in the KIDCAT study and 81.5% in the current study). All children in the KIDCAT study identified to have asymptomatic CVC-RT were treated with LMWH for 1 month3. In contrast, only 1 child in this study diagnosed with asymptomatic CVC-RT ultrasound received therapeutic anticoagulation. The KIDCAT study did not perform any follow-up.
Over 80% of this study cohort had UFH administered for thromboprophylaxis, at a median dose of 10u/kg/hr. A recent meta-analysis of thromboprophylaxis for CVC-RT in children reported that from 37 articles, the pooled frequency of thrombosis was 0.20 (95% CI 0.16 to 0.24).21 There was no evidence to suggest that thromboprophylaxis strategies reduced the risk of CVC-RT.21 However, missing data did limit the outcome data from the studies evaluated.21 The incidence of CVC-RT of 22% reported in this study is proportionate to the pooled frequency reported in the meta-analysis and indicative that UFH thromboprophylaxis at doses described is perhaps not protective against CVC-RT in children. This is consistent with data from an RCT comparing UFH thromboprophylaxis to placebo conducted in 90 children with a CVC, in which the incidence of thrombosis was 15% in the intervention group and 16% in placebo group.6
Twenty-four of the 32 children identified to have CVC-RT during admission, attended for follow-up; only 3 of these 24 children had residual thrombus at follow-up. The low incidence of residual asymptomatic CVC-RT 2 years after CVC placement supports previous evidence provided by 2 prospective studies, that many asymptomatic CVC-RT are transient.22,23 The rates of transient thrombosis reported by 2 studies conducted by Rudd et al are 22% (4 of 18) and 62.6% (10 of 16), all of which had resolved by the time of follow-up imaging 5-6 months later. None of the patients identified in these 2 studies received treatment.23,24 The findings from the current study together with this previous evidence suggest that a significant proportion of asymptomatic CVC-related thrombus are transient, resolve without treatment and cause no long-term sequelae for patients.
D-dimer and Factor-VIII have been laboratory parameters widely explored in both adults and children as predictors of thrombosis.10,25 Elevated D-dimer and factor VIII have been reported to be predictive of poor outcomes, such as PTS, in children with known thrombosis.25 The results of this prospective study demonstrate that neither D-dimer nor Factor-VIII were useful predictors of acute CVC-RT or clinically significant PTS in this cohort. However, this study identified that cardiac arrest was predictive of acute CVC-RT during admission and placement of the CVC into a femoral vein was predictive of residual CVC-RT at follow-up.
Five previous studies that examined the incidence of CVC-RT in critically-ill children report an increased incidence of CVC-RT in children with femoral CVC’s.1,7-9,15 A retrospective study of over 300 infants with CVC’s reported that femoral CVC’s were significantly associated with a higher incidence of thrombosis, compared with jugular and subclavian CVCs (P < .01).15 Two older studies of CVC-RT in critically-ill children report incidences of femoral CVC-RT of 21.7% in a cohort of 76 children and 35% in a cohort of 20 children.7,8 Talbott et al’s study prospectively followed children only with femoral CVCs and was limited by its small sample size; however, all cases of CVC-RT were asymptomatic.7 Comparatively, the study by Beck at al reported 10 of 17 children with CVC-RT were asymptomatic.
Children with femoral CVCs were identified as a sub-group in this study with multiple risk factors for thrombosis and overall higher acuity as evidenced by a significantly higher PIM2, elevated D-dimers and Factor-VIII levels. There was also a higher incidence of acute complications such as hypotension and cardiac arrest, compared with children with jugular CVCs. A cardiac arrest indicates a period of hypotension and low cardiac output. This creates a state of low flow through the extremities but also potentially through the central venous circulation. Any state of sustained low flow together with the presence of a CVC contributes to an increased risk of thrombosis.15 Whether these findings point to a difference in thrombosis risk associated with site of CVC placement or whether they reflect the increased thrombosis risk with increasing severity of systemic illness will need to be determined in subsequent studies.
In this study, there was a trend indicating that acute complications (cardiac arrest and major bleeding) may contribute to an increased risk of CVC-RT. In the Faustino study, which recruited a similar cohort in PICU and assessed for asymptomatic CVC-RT, acute complications were not associated with thrombosis.9 In a follow-up study, the same group developed a prediction model for CVC-RT identifying blood product transfusion and increasing age to be predictive of thrombosis.11 The definition of major bleeding used for this study, provided by the ISTH, stipulates that to be classified as having a major bleeding episode the child had to have received a blood product transfusion.26 Given blood product transfusion (including red blood cells, platelets, fresh frozen plasma and cryoprecipitate) is indicated to treat episodes of major or clinically relevant bleeding and that both the current study and the Marquez study have identified that bleeding and/or blood product transfusion increases the risk of CVC-RT, it appears that major bleeding and its associated treatment need to be considered as risk factors for CVC-RT in critically-ill children.
The MJI tool assessed clinically significant PTS in 1.6% of this cohort, despite 10.3% of children having some degree of PTS. Published estimates of PTS incidence in pediatric populations may overcall the clinical significance of this outcome measure. A study by Polen et al similarly reported differences in the diagnosed rates of PTS by the MV compared with the MJI, with the MJI identifying less cases of all PTS but more patients with clinically significant PTS.27 The differences in how the tools score PTS can be attributed to the value placed on subjective symptoms as opposed to the measure of objective signs. On the MJI tool, pain is scored on a scale of 1 to 5 and then limitations associated with pain are also scored. Comparatively, the MV only has 1 item about pain and thus pain is only reported as present or absent. Pain assessment becomes more complex when asking younger, preschool children about their past experiences of pain. Based on the findings of this study, MJI may overcall the incidence of clinically significant PTS in younger children. As the MV is less dependent on measuring pain, it may be more suitable for use in younger children.
There are some limitations to this study. During admission, 23% (n = 43) of eligible patients did not have their ultrasound performed and despite an extended follow-up period, 27% (n = 47) of eligible patients did not receive per protocol follow-up. However, some kind of follow-up, which was able to exclude major adverse events including death, was available on all but 1 patient.
The sample was intended to be heterogeneous to capture the natural history of asymptomatic CVC-RT in children and maximize the sample size. Thus our study had minimal exclusion criteria. However, we finished with a predominantly young cohort, mostly with CHD as the primary diagnosis, reflecting the patient flow in our PICU. This may limit the generalizability of the findings to other children with CVCs or those with critical illness. For young children, and particularly infants, the ratio of CVC diameter is much greater compared with the vessel diameter. This changes the rate of flow around the CVC and combined with inflammation, endothelial damage and the reliance on gravity flow can precipitate a thrombogenic response.15 Furthermore, children in this cohort had untunneled, short-term CVCs. Preliminary results from a recent study by Jaffray et al clearly distinguished a difference in risk of thrombosis for children with peripherally inserted central catheters versus those with tunneled CVCs.28
As has been suggested recently, adaption of a rare diseases model that recognizes many venous thromboses in children are in fact different pathophysiological entities united by common therapeutic options, may benefit advancement in this field.29 This no doubt applies to the variance in CVC-RT as well. Nonetheless, this is the first data from a cohort of children who did not receive any therapeutic anticoagulation to treat the asymptomatic CVC-RT and so does provide some limited basis for extrapolation until specific data from other pediatric CVC populations becomes available from future studies.
This study reported a similar rate of asymptomatic CVC-RT to previous studies, however provides the best natural history outcome data available because the presence or absence of asymptomatic thrombosis was blinded to the clinical team. The study demonstrated a low risk of short and long-term sequelae among those with thrombosis, despite those children not receiving any therapeutic anticoagulation. Outcome data for children with and without thrombosis did not differ. When compared with the known risks of anticoagulation in sick children, this would suggest that asymptomatic CVC-RT, even in critically unwell children might not require specific treatment.
The study highlights that there are clearly children with multiple risk factors for CVC-RT. Future studies are recommended to develop risk stratification methods that might then provide a more rational approach to thromboprophylaxis in critically-ill children. Furthermore, this study confirms the importance of consistency in the classification, diagnosis and reporting of clinically significant PTS in children, as reporting mild PTS may overcall the incidence of clinically relevant PTS in children. The study highlights the limitations of the MV and MJI tools and suggests that the tools may have differential value in different age groups of children. This should be a focus for future studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Susan Donath and Tania Griffiths for their contributions to data collection and analysis and Wincy Au-Yang for help with data entry.
This work was supported (in part) by research funding to Sophie Jones from the National Health and Medical Research Council (postgraduate scholarship), the Australian Nurses Council, and the Royal Children’s Hospital Foundation.
Authorship
Contribution: S.J. was responsible for study design, recruitment, all data collection, data analysis, and writing the manuscript; P.M. and F.N. supervised study design and data collection and analysis, and reviewed the manuscript; W.B. supervised recruitment and data collection and reviewed the manuscript; and T.C. reported ultrasounds and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sophie Jones, Department of Clinical Haematology, The Royal Children’s Hospital Melbourne, 50 Flemington Rd, Parkville, VIC 3052, Australia; e-mail: sophie.jones@rch.org.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal