In this issue of Blood, Camargo et al have identified 2 distinct functional subsets of cytomegalovirus (CMV) phosphoprotein 65 antigen peptide-specific, polyfunctional CD8+ T-cells that independently predict risk for clinically significant CMV DNAemia following allogeneic hematopoietic cell transplantation (HCT).1
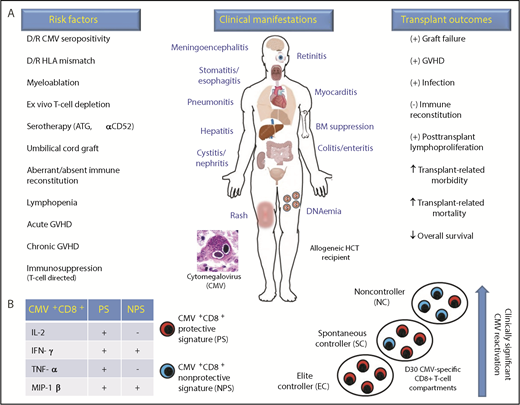
Effect of CMV and donor-derived CMV+CD8+ T-cell recovery in the allogeneic HCT recipient. (A) Risk factors, clinical manifestations, and transplant outcomes associated with CMV infection in the allogeneic HCT recipient. (B) PS and NPS of donor CMV (pp65 antigen peptide)-specific CD8+ T cells associate with low or high viral DNA levels (international units per milliliter) of clinically significant CMV DNAemia (reactivation), respectively. In addition, 3 separate HCT recipient subgroups were delineated based upon distinct donor CMV+CD8+ profiles: EC who experienced no CMV DNAemia following allogeneic HCT had the highest levels of PS; NC who experienced high levels of CMV DNAemia (>1000 IU/mL) and required preemptive antiviral therapy had the lowest levels of PS and highest levels of NPS; and SC who self-resolved episodes of low levels of CMV DNAemia (<200 IU/mL) without antiviral therapy had predominantly higher levels of PS than NPS. α, anti-; ATG, antithymocyte globulin; D, posttransplant day; D/R, donor/recipient; BM, bone marrow; HLA, human leukocyte antigen. +, promotes; -, inhibits.
Effect of CMV and donor-derived CMV+CD8+ T-cell recovery in the allogeneic HCT recipient. (A) Risk factors, clinical manifestations, and transplant outcomes associated with CMV infection in the allogeneic HCT recipient. (B) PS and NPS of donor CMV (pp65 antigen peptide)-specific CD8+ T cells associate with low or high viral DNA levels (international units per milliliter) of clinically significant CMV DNAemia (reactivation), respectively. In addition, 3 separate HCT recipient subgroups were delineated based upon distinct donor CMV+CD8+ profiles: EC who experienced no CMV DNAemia following allogeneic HCT had the highest levels of PS; NC who experienced high levels of CMV DNAemia (>1000 IU/mL) and required preemptive antiviral therapy had the lowest levels of PS and highest levels of NPS; and SC who self-resolved episodes of low levels of CMV DNAemia (<200 IU/mL) without antiviral therapy had predominantly higher levels of PS than NPS. α, anti-; ATG, antithymocyte globulin; D, posttransplant day; D/R, donor/recipient; BM, bone marrow; HLA, human leukocyte antigen. +, promotes; -, inhibits.
Donor-derived, protective signature (PS; interleukin-2 [IL-2]+ interferon-γ [IFN-γ]+ tumor necrosis factor-α [TNF-α]+ macrophage inflammatory protein-1β [MIP-1β]+) and nonprotective signature (NPS; IL-2+IFN-γ−TNF-α+MIP-1β−) CMV+CD8+ profiles associated with decreased and increased risk for CMV DNAemia, respectively. Furthermore, the 42 adult allogeneic HCT recipients were categorized into 3 distinct subgroups based upon PS and NPS profiles: elite controllers (EC) did not have CMV DNAemia and had the highest PS levels; noncontrollers (NC) experienced clinically significant CMV DNAemia requiring antiviral therapy and had the highest NPS and lowest PS levels; and spontaneous controllers (SC) cleared low-level CMV DNAemia without need for antiviral therapy and had higher NPS levels than EC, but lower than NC. In summary, this methodical work defines CMV-specific CD8+ functional profiles that provide more robust prediction for CMV risk than either absolute numbers of lymphocytes or IFN-γ production.
CMV is the most significant double-stranded DNA virus in the allogeneic HCT setting and commonly manifests as DNAemia in the early post-HCT period.2 High plasma viral DNA levels as detected by quantitative polymerase chain reaction associate with increased risk for organ disease, resulting in increased nonrelapse mortality and decreased overall survival (see figure).3 Consortium studies from the European Society for Blood and Marrow Transplant4 and the Center for International Blood and Marrow Transplant Research5 corroborate the negative effect of CMV on posttransplant overall survival in the contemporary transplant era. CMV increases nonrelapse mortality through its association with other viral, bacterial and fungal infections, graft-versus-host disease (GVHD), and posttransplant lymphoproliferation and its induction of organ toxicity through direct cytotoxicity and antiviral therapies used to treat CMV infections (see figure). Together, these detrimental effects of CMV infection and its associated therapy substantially increase health care resource utilization.6
Host innate and adaptive immune responses mediate protection against CMV, and defining protective immune responses has translated into novel approaches for diagnosing and treating CMV infection following allogeneic HCT.7 Immune-based assays have emerged to define CMV infection risk and timing for both preemptive and prophylactic antiviral therapy.8 Similarly, viral-specific T cells have emerged as an effective form of adoptive cellular therapy against CMV infection9 and may ultimately become a cost-effective alternative to antiviral therapies,10 which have reduced efficacy and more significant toxicity.
Camargo et al impart further translational insight into adaptive immune responses conferring protection against CMV following allogeneic HCT. Their work shows that CMV reactivation results from loss of polyfunctionality within the CMV-specific CD8+ T-cell compartment. Using 13-color flow cytometry performed on peripheral blood mononuclear cells obtained on day 30 postallogeneic HCT in 42 adult patients, the authors identified donor-derived CMV+CD8+ PS and NPS biomarkers that significantly predicted CMV reactivation (DNAemia) independent of steroid use and in vivo T-cell depletion with antithymocyte globulin serotherapy (see figure). In addition, CMV+CD8+ T-cell profiles enabled patient subgroupings, within which clinically significant CMV risk associated with levels of PS and NPS.
Given these novel findings, it is important that the study cohort reflects published incidences of CMV reactivation and disease. Of the original 56 CMV seropositive adult allogeneic HCT patients, 14 were excluded from deep functional analysis because of lymphopenia. However, rates of CMV reactivation were similar between excluded (n = 14 patients, 57% CMV DNAemia) and analyzed (n = 42, 69% CMV DNAemia) patients. Also, overall incidence of CMV disease in the study was low (n = 4 patients, overall CMV disease rate <10%); therefore, the study rates for CMV reactivation and disease were consistent with those reported in the transplant literature11 and were not affected by excluding patients with lymphopenia. Did the patient cohort with the highest NPS profile (NC) also have other CMV-associated complications such as graft failure and bacterial and fungal superinfections? Although engraftment did not differ among the 3 patient subgroups, 12-month cumulative incidence of invasive fungal and bacterial infections were threefold higher in the NC vs EC and SC subgroups, providing further validity for the study cohort analyzed.
Equally as important as a representative study cohort is to ensure that sample acquisition and subsequent immunophenotype analyses were not confounded by effects on immune reconstitution, such as acute GVHD. For the analyzed study cohort, the median time to CMV reactivation was 30 days (interquartile range, 12-35 days), and the median time to acute GVHD was 108 days (interquartile range, 43-123). Importantly, all associations with CMV+CD8+ PS and NPS profiles with respect to cumulative incidence and hazard ratios for CMV were obtained from analyses restricted to patients in whom CMV reactivation occurred after peripheral blood mononuclear cell sample acquisition.
The current study does have inherent limitations, including its small sample size and heterogeneity in transplant-related factors that can affect donor-derived immune reconstitution. In addition, other DNA viral infections that concomitantly occur with CMV reactivation and allograft immune cell content were not studied, which also could influence study results. Last, whether the reported CMV+CD8+ profiles are observed in younger patients in whom central thymic T-cell reconstitution pathways are intact remains unanswered. Yet, despite these shortcomings, Camargo et al provide convincing data that, if prospectively validated in a larger cohort of patients, have the potential to apply a readily available technique across transplant centers. This could standardize monitoring CMV immune reconstitution to direct clinical decisions such as initiation and duration of prophylactic and preemptive antiviral therapy and the use of preemptive cellular therapy before onset of GVHD and its associated T cell–directed immunosuppression. Such a standardized approach to CMV would be warmly embraced by the transplant, cell therapy, and infectious diseases communities.
Conflict-of-interest disclosure: J.J.A. reports medical consultancy for MORE Health.