Key Points
Among 748 662 Medicare beneficiaries, the risk of arterial thromboembolic events was increased 69% in the year before cancer diagnosis.
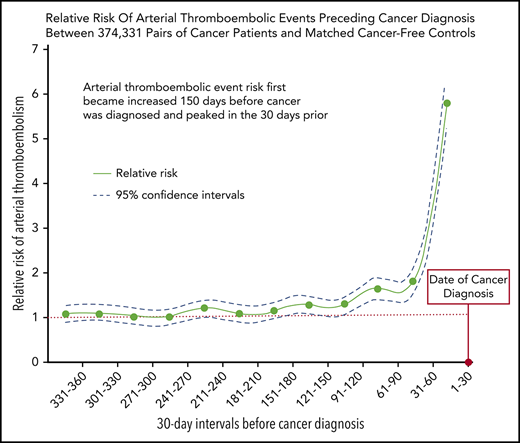
The increased risk of arterial thromboembolic events began 5 months before cancer was officially diagnosed and peaked in the month prior.
Abstract
Cancer patients face an increased risk of arterial thromboembolism; however, it is uncertain when this excess risk begins. This study evaluated the risk of arterial thromboembolism before cancer diagnosis. Using the population-based Surveillance Epidemiology and End Results-Medicare linked dataset, we identified 374 331 patients ≥67 years of age with a new primary diagnosis of breast, lung, prostate, colorectal, bladder, uterine, pancreatic, gastric cancer, or non-Hodgkin lymphoma from 2005 through 2013. Cancer patients were individually matched by demographics and comorbidities to Medicare beneficiaries without cancer, who served as controls. Validated diagnosis codes were used to identify arterial thromboembolic events, defined as a composite of myocardial infarction or ischemic stroke. The Mantel-Haenszel estimator was used to compare risks of arterial thromboembolic events between cancer and noncancer groups during 30-day periods in the 360 days before date of cancer diagnosis. From 360 to 151 days before cancer diagnosis, the 30-day interval risks of arterial thromboembolic events were similar between cancer patients and matched controls. From 150 to 1 day before cancer diagnosis, the interval 30-day risks of arterial thromboembolic events were higher in cancer patients vs matched controls, progressively increasing as the cancer diagnosis date approached and peaking during the 30 days immediately before cancer diagnosis, when 2313 (0.62%) cancer patients were diagnosed with an arterial thromboembolic event vs 413 (0.11%) controls (odds ratio, 5.63; 95% confidence interval, 5.07-6.25). In conclusion, the risk of arterial thromboembolic events begins to increase 150 days before the date of cancer diagnosis in older persons and peaks in the 30 days before.
Introduction
An American’s lifetime incidence of cancer is estimated to be 39%.1 In the 6 months after diagnosis, cancer patients are twice as likely to develop myocardial infarction and ischemic stroke compared with matched controls without cancer.2 Some of this excess risk may be due to external factors associated with cancer, such as the effects of stress and cancer treatments, frequent interruption of antithrombotics, and increased surveillance.3 However, cancer itself, through effects on hemostatic and fibrinolytic pathways, may be the biggest driver of thromboembolism risk, as evidenced by the wide variability in risk by cancer type and stage.2,4-6 Cancer’s prothrombotic effects include hematogenous release of cancer-derived microparticles that trigger the coagulation cascade, production of procoagulant factors such as factor X, release of mucins that activate platelets and endothelial cells through binding of P-selectin, and stimulation of neutrophils to release decondensed chromatin that forms prothrombotic neutrophil extracellular traps.3,7,8 These effects, although linked to overall tumor burden, may precede cancer diagnosis; thus, it is speculated that some cancers can disrupt the body’s hemostatic system and produce thrombosis months or even years before detection.9 For example, up to 10% of venous thromboembolism may be associated with occult cancer10 ; therefore, venous thromboembolism, especially if unprovoked and in older patients, can initiate a malignancy screen, although this practice is not endorsed in current guidelines because of a lack of proven benefit in large, randomized trials.11,12
Conversely, the association between arterial thromboembolism and occult cancer is less certain, and although there are numerous case reports describing ischemic stroke or myocardial infarction serving as the initial presentation of cancer, there are few large-scale, population-based studies detailing the risks of arterial thromboembolism in the months before the pathological diagnosis of cancer.13-17 Therefore, we performed a retrospective, matched case-control study evaluating the risks of arterial thromboembolic events in the year before cancer detection. Because there may be a protracted time of biological activity between the development of cancer and its recognition, we hypothesized that the risks of arterial thromboembolic events increase several months before the pathological diagnosis of cancer.
Materials and methods
Design
We used linked data from the Surveillance, Epidemiology, and End Results (SEER) registry and Medicare claims from 2004 through 2013 to evaluate the risks of arterial thromboembolic events in the year before a diagnosis of cancer. The SEER-Medicare program comprises multiple population-based cancer registries in the United States, which are linked to Medicare enrollment and claims files, providing detailed clinical information about a diverse population of cancer patients.18 SEER includes ∼28% of all patients diagnosed with cancer in the United States.18 It also provides data from a 5% random sample of Medicare beneficiaries without cancer who live in SEER geographic areas, which enabled us to compare risks of arterial thromboembolic events between patients with incident cancer and control patients without cancer.18 The Medicare claims data used for this analysis included the physician and supplier file, the outpatient standard analytical file, and the provider analysis and review file. B.B.N. had full access to all the data in the study and takes responsibility for its integrity and the data analysis. The Memorial Sloan Kettering institutional review board approved this study as exempt research and waived the need for informed consent.
Population
SEER data are organized, and accessed, by individual cancer site, making it impractical to analyze all cancer patients registered in SEER. Therefore, for this analysis, we restricted our cancer population to patients ≥67 years of age diagnosed with breast, lung, prostate, colorectal, bladder, non-Hodgkin lymphoma, uterine, pancreatic, or gastric cancers from 1 January 2005 to 31 December 2013. These cancer sites were selected because they include the 5 most common (overall and solid tumor) cancers, the most common hematological cancer, the most common gynecological cancer, and the 2 cancers historically most associated with thromboembolism (pancreatic and gastric).2,19 In total, these cancers account for approximately two-thirds of all incident cancer diagnoses in the United States.1 If patients were diagnosed with multiple primary cancers during the study period, they were assigned the cancer site diagnosed first. We excluded patients younger than 67 years of age because most people in the United States first become eligible for Medicare after they turn 65, and we required sufficient time to evaluate comorbidities in the 730 to 365 days before study entry.
As done previously, we used site record definitions from the SEER Patient Entitlement and Diagnosis Summary File, as well as the International Classification of Diseases (ICD) for Oncology, third edition, site recode classification to identify the cancer population.2,4 The specific site definitions and recodes used were: breast (site C500-509; recode 26000); lung (sites C300-C301, C310-C319, C320-C329, C339, C340-C349, C381-383, C384, C388, C390, C398, C399; recodes 22010, 22020, 22030, 22050, 22060); prostate (site C619; recode 28010); colorectal (sites C180-C189, C199, C209, C210-C212, C218, C260; recodes 21041-21049, 21051, 21052, 21060); bladder (sites C670-679; recode 29010); non-Hodgkin lymphoma (sites C024, C098, C099, C111, C142, C379, C422, C770-779; recodes 33041, 33042); uterine (sites C540-549; recodes 27020, 27030); pancreas (sites C250-259; recode 21100); and gastric (sites C160-C169; recode 21020).
Cancer patients were excluded if they lacked Part A or B Medicare coverage, belonged to a health maintenance organization in the 2 years before their cancer diagnosis, their month of cancer diagnosis was missing, their age at the time of cancer diagnosis was <67 years, or we could not identify a control patient without cancer to match. To minimize misclassification error and to focus on first arterial thromboembolic events, we also excluded patients if they had an ICD, ninth edition, Clinical Modification (ICD-9-CM) diagnosis code for cerebrovascular disease (430.xx-438.xx) or coronary heart disease (410.xx-414.xx) 730 to 365 days before their date of cancer diagnosis.
All cancer patients were individually matched to a control patient without cancer by year of birth, sex, race (white or nonwhite), SEER registry (surrogate for geographic region), Charlson comorbidity index (trichotomized into 0, 1, and ≥2), and the diagnoses of hypertension (ICD-9-CM codes 401.xx-405.xx, 437.2) and atrial fibrillation (ICD-9-CM codes 427.31, 427.32) in the 730 to 365 days before study entry; these latter diagnoses were individually matched because they are important cardiovascular risk factors missing from the Charlson comorbidity index.20 Similar to cancer cases, we excluded control patients if they lacked Medicare Part A or B coverage, belonged to a health maintenance organization in the 2 years before study entry, or they had a Medicare claim for cerebrovascular or coronary heart disease 730 to 365 days before their date of study entry.
Measurements
We defined an arterial thromboembolic event as the composite of myocardial infarction or ischemic stroke. We also assessed myocardial infarction alone and ischemic stroke alone. Validated ICD-9-CM diagnostic code algorithms were used to identify these events.21,22 Myocardial infarction was identified by inpatient code 410.xx in any diagnostic position.21 Ischemic stroke was identified by inpatient codes 433.x1, 434.x1, or 436 in any diagnostic position without concurrent codes for rehabilitation (V57) in the primary diagnostic position or trauma (800.xx-804.xx, 850.xx-854.xx) or hemorrhagic stroke (430.xx, 431.xx) in any diagnostic position.22 These diagnostic code algorithms have been previously shown to have ≥90% positive predictive value for correctly identifying myocardial infarction and ischemic stroke when compared with detailed chart review.21,22 Mesenteric ischemia and systemic artery thromboembolism were not ascertained for the primary analysis because these are rare diseases that lack reliable diagnostic codes for identification through claims data.23
Statistical analysis
Descriptive statistics with exact confidence intervals (CIs) were used to describe patient characteristics at the time of study entry, which was defined as the date of cancer diagnosis for both cancer patients and their matched controls without cancer. Risks of arterial thromboembolic events were compared between groups during 1-month periods in the 12 months before study entry. The Mantel-Haenszel estimator for matched data was used to calculate odds ratios (ORs) and absolute risks for each 1-month period.
Several sensitivity analyses were performed. First, to account for the unknown exact date of cancer diagnosis within SEER (only month and year are provided; therefore, per standard practice, we used the middle of the month for analyses), we excluded events that occurred during the 15 days immediately before the recorded date of cancer diagnosis. Second, to account for the potential confounding effects of withholding anticoagulation for biopsy, we excluded patients with a diagnosis of atrial fibrillation. Third, to account for an imbalance in the rate of chronic obstructive pulmonary disease between groups, we matched cancer patients to cancer-free controls by this diagnosis (ICD-9-CM codes 490.xx-492.xx, 494.xx, 496.xx), in addition to the variables previously matched by in our primary analysis.
In secondary analysis, ORs and absolute risks were stratified by cancer stage at diagnosis. As in prior SEER-Medicare studies, cancer staging was defined according to the American Joint Committee on Cancer classification, except for prostate cancer, which was staged according to the T (clinical) classification, and non-Hodgkin lymphoma, which was staged according to the Ann Arbor classification.2,24-26 Patients with unknown cancer stage (and their matched controls) were excluded from this secondary analysis.
In a post hoc exploratory analysis, we expanded the arterial thromboembolic event composite outcome to include systemic artery thromboembolism and acute mesenteric ischemia, in addition to acute myocardial infarction and ischemic stroke. Systemic artery thromboembolism was identified by inpatient code 444.xx in any diagnostic position. Acute mesenteric ischemia was identified by inpatient code 557.0 in any diagnostic position. An α error of 5% was used to determine statistical significance. All analyses were performed by A.S.R. using SAS (version 9.4, SAS Institute Inc.) or R (version 3.4.3) software.
Results
Baseline characteristics
We identified 374 331 pairs of cancer patients and matched controls without cancer who met study eligibility criteria. The mean age was 76 years (standard deviation [SD], 7) and 52% were women (Table 1). Prostate (n = 82 099), breast (n = 74 638), lung (n = 74 633), and colorectal (n = 56 366) cancers accounted for 77% of cancer types. At diagnosis, 30% of cancers were stages III or IV.
Characteristics of pairs of cancer patients and matched controls, 2004-2013
| Characteristic . | Cancer patients (n = 374 331)* . | Controls without cancer (n = 374 331) . |
|---|---|---|
| Age, mean (SD), y | 76 (7) | 76 (7) |
| Sex | ||
| Women | 193 726 (52) | 193 726 (52) |
| Men | 180 605 (48) | 180 605 (48) |
| Race | ||
| White | 314 387 (84) | 314 387 (84) |
| Nonwhite | 59 944 (16) | 59 944 (16) |
| Geographic region | ||
| Northeast | 75 013 (20) | 75 013 (20) |
| Midwest | 61 900 (17) | 61 900 (17) |
| South | 96 606 (26) | 96 606 (26) |
| West | 140 812 (38) | 140 812 (38) |
| Charlson comorbidities | ||
| 0 | 260 814 (70) | 260 814 (70) |
| 1 | 79 136 (21) | 79 136 (21) |
| 2 or more | 34 381 (9) | 34 381 (9) |
| Hypertension | 221 127 (59) | 221 127 (59) |
| Atrial fibrillation | 16 426 (4) | 16 426 (4) |
| Diabetes mellitus | 92 915 (25) | 96 338 (26) |
| Peripheral vascular disease | 36 444 (10) | 35 463 (9) |
| COPD | 54 300 (15) | 42 736 (11) |
| Congestive heart failure | 15 458 (4) | 15 537 (4) |
| Cardiac valvular disease | 27 287 (7) | 29 352 (8) |
| Liver disease | 1 481 (0.4) | 1 592 (0.4) |
| Chronic kidney disease | 14 218 (4) | 14 405 (4) |
| Census tract poverty level <10%† | 182 239 (49) | NA |
| Cancer type | ||
| Breast | 74 638 (20) | NA |
| Lung | 74 633 (20) | NA |
| Prostate | 82 099 (22) | NA |
| Colorectal | 56 366 (15) | NA |
| Bladder | 26 503 (7) | NA |
| Non-Hodgkin lymphoma | 21 560 (6) | NA |
| Uterine | 13 473 (4) | NA |
| Pancreatic | 16 386 (4) | NA |
| Gastric | 8 673 (2) | NA |
| Cancer stage‡ | ||
| 0 | 26 685 (7) | NA |
| 1 | 115 783 (31) | NA |
| 2 | 72 636 (19) | NA |
| 3 | 44 854 (12) | NA |
| 4 | 67 121 (18) | NA |
| Unknown | 47 252 (13) | NA |
| Characteristic . | Cancer patients (n = 374 331)* . | Controls without cancer (n = 374 331) . |
|---|---|---|
| Age, mean (SD), y | 76 (7) | 76 (7) |
| Sex | ||
| Women | 193 726 (52) | 193 726 (52) |
| Men | 180 605 (48) | 180 605 (48) |
| Race | ||
| White | 314 387 (84) | 314 387 (84) |
| Nonwhite | 59 944 (16) | 59 944 (16) |
| Geographic region | ||
| Northeast | 75 013 (20) | 75 013 (20) |
| Midwest | 61 900 (17) | 61 900 (17) |
| South | 96 606 (26) | 96 606 (26) |
| West | 140 812 (38) | 140 812 (38) |
| Charlson comorbidities | ||
| 0 | 260 814 (70) | 260 814 (70) |
| 1 | 79 136 (21) | 79 136 (21) |
| 2 or more | 34 381 (9) | 34 381 (9) |
| Hypertension | 221 127 (59) | 221 127 (59) |
| Atrial fibrillation | 16 426 (4) | 16 426 (4) |
| Diabetes mellitus | 92 915 (25) | 96 338 (26) |
| Peripheral vascular disease | 36 444 (10) | 35 463 (9) |
| COPD | 54 300 (15) | 42 736 (11) |
| Congestive heart failure | 15 458 (4) | 15 537 (4) |
| Cardiac valvular disease | 27 287 (7) | 29 352 (8) |
| Liver disease | 1 481 (0.4) | 1 592 (0.4) |
| Chronic kidney disease | 14 218 (4) | 14 405 (4) |
| Census tract poverty level <10%† | 182 239 (49) | NA |
| Cancer type | ||
| Breast | 74 638 (20) | NA |
| Lung | 74 633 (20) | NA |
| Prostate | 82 099 (22) | NA |
| Colorectal | 56 366 (15) | NA |
| Bladder | 26 503 (7) | NA |
| Non-Hodgkin lymphoma | 21 560 (6) | NA |
| Uterine | 13 473 (4) | NA |
| Pancreatic | 16 386 (4) | NA |
| Gastric | 8 673 (2) | NA |
| Cancer stage‡ | ||
| 0 | 26 685 (7) | NA |
| 1 | 115 783 (31) | NA |
| 2 | 72 636 (19) | NA |
| 3 | 44 854 (12) | NA |
| 4 | 67 121 (18) | NA |
| Unknown | 47 252 (13) | NA |
Data presented as number (%) unless otherwise specified.
COPD, chronic obstructive pulmonary disease; NA, not applicable.
Because of rounding, some values do not add up to 100.
Number and proportion of patients who live in areas where <10% of people are below the poverty line according to the 2000 census.
Refers to the American Joint Committee on Cancer, sixth edition, staging schema, except for patients with prostate cancer, who were staged according to the T (clinical) staging classification; patients with non-Hodgkin lymphoma were staged according to the Ann Arbor staging classification.
Primary analysis
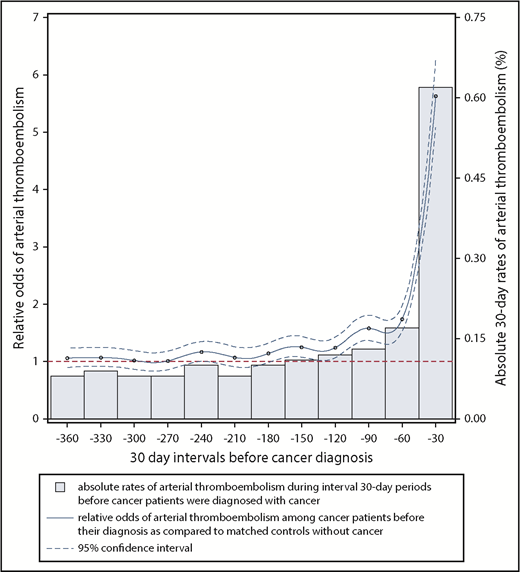
From 360 to 151 days before cancer diagnosis, the 30-day interval risks of arterial thromboembolic events were similar between cancer patients and matched controls without cancer (Figure 1; Table 2). From 150 to 1 day before cancer diagnosis, the interval 30-day risks of arterial thromboembolic events were higher in cancer patients vs matched controls, progressively increasing as the cancer diagnosis date approached, and peaking during the 30 days immediately before cancer diagnosis, when 2313 (0.62%) cancer patients were diagnosed with an arterial thromboembolic event vs 413 (0.11%) cancer-free controls (OR, 5.63; 95% CI, 5.07-6.25; P < .001). When analyzing all 360 days before study entry, 6567 (1.75%) cancer patients were diagnosed with an arterial thromboembolic event vs 3916 (1.05%) cancer-free controls (OR, 1.69; 95% CI, 1.63-1.76; P < .001). Among cancer patients who had an arterial thromboembolic event in the 360 days before cancer diagnosis, the primary underlying cancer sites were lung (n = 1908, 29%), colorectal (n = 1561, 24%), prostate (n = 752, 11%), breast (n = 663, 10%), bladder (n = 533, 8%), non-Hodgkin lymphoma (n = 384, 6%), pancreatic (n = 333, 5%), gastric (n = 250, 4%), and uterine (n = 183, 3%) (Table 3).
Relative and absolute odds of an arterial thromboembolic event during 30-day intervals in the 360 days before cancer diagnosis. The comparator group for the relative odds calculation was control patients without cancer who were individually matched to cancer cases by age, sex, race, geographic region, Charlson comorbidity index, history of hypertension, and history of atrial fibrillation. Arterial thromboembolic events were defined as a composite of myocardial infarction and ischemic stroke. Dashed blue lines, 95% CIs for the relative odds of arterial thromboembolic events.
Relative and absolute odds of an arterial thromboembolic event during 30-day intervals in the 360 days before cancer diagnosis. The comparator group for the relative odds calculation was control patients without cancer who were individually matched to cancer cases by age, sex, race, geographic region, Charlson comorbidity index, history of hypertension, and history of atrial fibrillation. Arterial thromboembolic events were defined as a composite of myocardial infarction and ischemic stroke. Dashed blue lines, 95% CIs for the relative odds of arterial thromboembolic events.
Risk of arterial thromboembolic events* before cancer diagnosis
| Time before cancer . | Cancer patients . | Cancer-free controls . | . | . |
|---|---|---|---|---|
| diagnosis, d . | Events, no. (%) . | Events, no. (%) . | OR (95% CI) . | P . |
| 1-30 | 2313 (0.62) | 413 (0.11) | 5.63 (5.07-6.25) | <.001 |
| 31-60 | 635 (0.17) | 365 (0.10) | 1.74 (1.53-1.98) | <.001 |
| 61-90 | 496 (0.13) | 315 (0.08) | 1.58 (1.37-1.81) | <.001 |
| 91-120 | 431 (0.12) | 349 (0.09) | 1.24 (1.07-1.42) | .003 |
| 121-150 | 427 (0.11) | 342 (0.09) | 1.25 (1.08-1.44) | .002 |
| 151-180 | 379 (0.10) | 333 (0.09) | 1.14 (0.98-1.32) | .08 |
| 181-210 | 312 (0.08) | 291 (0.08) | 1.07 (0.91-1.26) | .39 |
| 211-240 | 370 (0.10) | 318 (0.08) | 1.17 (1.00-1.35) | .05 |
| 241-270 | 309 (0.08) | 306 (0.08) | 1.01 (0.86-1.18) | .90 |
| 271-300 | 316 (0.08) | 311 (0.08) | 1.02 (0.87-1.19) | .84 |
| 301-330 | 334 (0.09) | 313 (0.08) | 1.07 (0.92-1.25) | .41 |
| 331-360 | 317 (0.08) | 300 (0.08) | 1.06 (0.90-1.24) | .49 |
| Time before cancer . | Cancer patients . | Cancer-free controls . | . | . |
|---|---|---|---|---|
| diagnosis, d . | Events, no. (%) . | Events, no. (%) . | OR (95% CI) . | P . |
| 1-30 | 2313 (0.62) | 413 (0.11) | 5.63 (5.07-6.25) | <.001 |
| 31-60 | 635 (0.17) | 365 (0.10) | 1.74 (1.53-1.98) | <.001 |
| 61-90 | 496 (0.13) | 315 (0.08) | 1.58 (1.37-1.81) | <.001 |
| 91-120 | 431 (0.12) | 349 (0.09) | 1.24 (1.07-1.42) | .003 |
| 121-150 | 427 (0.11) | 342 (0.09) | 1.25 (1.08-1.44) | .002 |
| 151-180 | 379 (0.10) | 333 (0.09) | 1.14 (0.98-1.32) | .08 |
| 181-210 | 312 (0.08) | 291 (0.08) | 1.07 (0.91-1.26) | .39 |
| 211-240 | 370 (0.10) | 318 (0.08) | 1.17 (1.00-1.35) | .05 |
| 241-270 | 309 (0.08) | 306 (0.08) | 1.01 (0.86-1.18) | .90 |
| 271-300 | 316 (0.08) | 311 (0.08) | 1.02 (0.87-1.19) | .84 |
| 301-330 | 334 (0.09) | 313 (0.08) | 1.07 (0.92-1.25) | .41 |
| 331-360 | 317 (0.08) | 300 (0.08) | 1.06 (0.90-1.24) | .49 |
Composite of myocardial infarction and ischemic stroke diagnoses.
Characteristics of cancer patients stratified by the diagnosis of arterial thromboembolic events in the year before cancer diagnosis
| Characteristic . | Preceding arterial thromboembolic event (n = 6567)* . | No preceding arterial thromboembolic event (n = 367 764) . |
|---|---|---|
| Age, mean (SD), y | 79 (7) | 76 (7) |
| Sex | ||
| Women | 3308 (50) | 190 418 (52) |
| Men | 3259 (50) | 177 346 (48) |
| Race | ||
| White | 5402 (82) | 308 985 (84) |
| Nonwhite | 1165 (18) | 58 779 (16) |
| Geographic region | ||
| Northeast | 1522 (23) | 73 491 (20) |
| Midwest | 1061 (16) | 60 839 (17) |
| South | 1844 (28) | 94 762 (26) |
| West | 2140 (33) | 138 672 (38) |
| Charlson comorbidities | ||
| 0 | 4121 (63) | 256 693 (70) |
| 1 | 1480 (23) | 77 656 (21) |
| ≥2 | 966 (15) | 33 415 (9) |
| Hypertension | 3836 (58) | 217 291 (59) |
| Atrial fibrillation | 377 (6) | 16 049 (4) |
| Diabetes mellitus | 1,95 (27) | 91 120 (25) |
| Peripheral vascular disease | 917 (14) | 35 527 (10) |
| COPD | 1183 (18) | 53 117 (14) |
| Congestive heart failure | 482 (7) | 14 976 (4) |
| Cardiac valvular disease | 533 (8) | 26 754 (7) |
| Liver disease | 20 (0.3) | 1 461 (0.4) |
| Chronic kidney disease | 386 (6) | 13 832 (4) |
| Census tract poverty level <10%† | 2895 (44) | 179 344 (49) |
| Cancer type | ||
| Breast | 663 (10) | 73 975 (20) |
| Lung | 1908 (29) | 72 725 (20) |
| Prostate | 752 (11) | 81 347 (22) |
| Colorectal | 1561 (24) | 54 805 (15) |
| Bladder | 533 (8) | 25 970 (7) |
| Non-Hodgkin lymphoma | 384 (6) | 21 176 (6) |
| Uterine | 183 (3) | 13 290 (4) |
| Pancreatic | 333 (5) | 16 053 (4) |
| Gastric | 250 (4) | 8 423 (2) |
| Cancer stage‡ | ||
| 0 | 364 (6) | 26 321 (7) |
| I | 1507 (23) | 114 276 (31) |
| II | 1065 (16) | 71 571 (19) |
| III | 1044 (16) | 43 810 (12) |
| IV | 1596 (24) | 65 525 (18) |
| Unknown | 991 (15) | 46 261 (13) |
| Characteristic . | Preceding arterial thromboembolic event (n = 6567)* . | No preceding arterial thromboembolic event (n = 367 764) . |
|---|---|---|
| Age, mean (SD), y | 79 (7) | 76 (7) |
| Sex | ||
| Women | 3308 (50) | 190 418 (52) |
| Men | 3259 (50) | 177 346 (48) |
| Race | ||
| White | 5402 (82) | 308 985 (84) |
| Nonwhite | 1165 (18) | 58 779 (16) |
| Geographic region | ||
| Northeast | 1522 (23) | 73 491 (20) |
| Midwest | 1061 (16) | 60 839 (17) |
| South | 1844 (28) | 94 762 (26) |
| West | 2140 (33) | 138 672 (38) |
| Charlson comorbidities | ||
| 0 | 4121 (63) | 256 693 (70) |
| 1 | 1480 (23) | 77 656 (21) |
| ≥2 | 966 (15) | 33 415 (9) |
| Hypertension | 3836 (58) | 217 291 (59) |
| Atrial fibrillation | 377 (6) | 16 049 (4) |
| Diabetes mellitus | 1,95 (27) | 91 120 (25) |
| Peripheral vascular disease | 917 (14) | 35 527 (10) |
| COPD | 1183 (18) | 53 117 (14) |
| Congestive heart failure | 482 (7) | 14 976 (4) |
| Cardiac valvular disease | 533 (8) | 26 754 (7) |
| Liver disease | 20 (0.3) | 1 461 (0.4) |
| Chronic kidney disease | 386 (6) | 13 832 (4) |
| Census tract poverty level <10%† | 2895 (44) | 179 344 (49) |
| Cancer type | ||
| Breast | 663 (10) | 73 975 (20) |
| Lung | 1908 (29) | 72 725 (20) |
| Prostate | 752 (11) | 81 347 (22) |
| Colorectal | 1561 (24) | 54 805 (15) |
| Bladder | 533 (8) | 25 970 (7) |
| Non-Hodgkin lymphoma | 384 (6) | 21 176 (6) |
| Uterine | 183 (3) | 13 290 (4) |
| Pancreatic | 333 (5) | 16 053 (4) |
| Gastric | 250 (4) | 8 423 (2) |
| Cancer stage‡ | ||
| 0 | 364 (6) | 26 321 (7) |
| I | 1507 (23) | 114 276 (31) |
| II | 1065 (16) | 71 571 (19) |
| III | 1044 (16) | 43 810 (12) |
| IV | 1596 (24) | 65 525 (18) |
| Unknown | 991 (15) | 46 261 (13) |
Data presented as number (%) unless otherwise specified.
Because of rounding, some values do not add up to 100.
Number and proportion of patients who live in areas where <10% of people are below the poverty line according to the 2000 census.
Refers to the American Joint Committee on Cancer, sixth edition, staging schema, except for patients with prostate cancer, who were staged according to the T (clinical) staging classification; patients with non-Hodgkin lymphoma were staged according to the Ann Arbor staging classification.
Sensitivity analysis
When excluding events that occurred during the 15 days immediately before the recorded date of cancer diagnosis (ie, analysis restricted to 16-360 days before study entry), risk differences between groups were attenuated but still significant with 4814 (1.29%) arterial thromboembolic events diagnosed in cancer patients vs 3718 (0.99%) events in cancer-free controls (OR, 1.31; 95% CI, 1.25-1.37; P < .001). In the 16 to 30 days before study entry, 561 (0.15%) cancer patients were diagnosed with an arterial thromboembolic event vs 215 (0.06%) cancer-free controls (OR, 2.62; 95% CI, 2.24-3.07; P < .001).
When restricted to patients without a diagnosis of atrial fibrillation (357 905 pairs of patients), in the 360 days before study entry, 6190 (1.73%) cancer patients were diagnosed with an arterial thromboembolic event vs 3633 (1.02%) cancer-free controls. In these patients, the risk of an arterial thromboembolic event first became increased 150 days before the date of cancer diagnosis and thereafter progressively increased until peaking in the 30 days preceding the diagnosis (OR, 5.77; 95% CI, 5.18-6.44).
When cancer patients and their cancer-free controls were matched by the diagnosis of chronic obstructive pulmonary disease in addition to the variables previously matched by in our primary analysis (298 459 pairs of patients), there remained an increased risk of arterial thromboembolic events in the 360 days before the date of cancer diagnosis (OR, 1.91; 95% CI, 1.82-2.00; P < .001). This risk first became consistently increased at 150 days before the date of cancer diagnosis and thereafter progressively increased until peaking in the 30 days preceding the diagnosis (OR, 6.30; 95% CI, 5.56-7.14).
Secondary analyses
Cancer patients’ relative risks for myocardial infarction and ischemic stroke in isolation were similar, although their absolute risk for myocardial infarction appeared slightly higher than for ischemic stroke (Table 4). The risks of both myocardial infarction and ischemic stroke were mostly equal between cancer patients and cancer-free controls until about 150 days before cancer diagnosis, when risks became higher among cancer patients and progressively increased until the date of cancer diagnosis. In the 360 days before study entry, there were 3877 (1.04%) myocardial infarctions diagnosed in cancer patients vs 2171 (0.58%) in cancer-free controls (OR, 1.80; 95% CI, 1.71-1.90; P < .001). Meanwhile, during this 360-day period, there were 2913 (0.78%) ischemic strokes diagnosed in cancer patients vs 1836 (0.49%) in cancer-free controls (OR, 1.59; 95% CI, 1.50-1.69; P < .001).
Risks of myocardial infarction and ischemic stroke before cancer diagnosis
| Period before cancer diagnosis, d . | Cancer patients . | Cancer-free controls . | . | . |
|---|---|---|---|---|
| Outcomes, no. (%) . | Outcomes, no. (%) . | OR (95% CI) . | P . | |
| Myocardial infarction | ||||
| 1-30 | 1408 (0.38) | 220 (0.06) | 6.43 (5.57-7.41) | <.001 |
| 31-60 | 340 (0.09) | 201 (0.05) | 1.69 (1.42-2.01) | <.001 |
| 61-90 | 274 (0.07) | 170 (0.05) | 1.61 (1.33-1.95) | <.001 |
| 91-120 | 241 (0.06) | 187 (0.05) | 1.29 (1.07-1.56) | .009 |
| 121-150 | 237 (0.06) | 192 (0.05) | 1.24 (1.02-1.50) | .03 |
| 151-180 | 222 (0.06) | 200 (0.0%) | 1.11 (0.92-1.34) | .28 |
| 181-210 | 170 (0.05) | 170 (0.05) | 1.00 (0.81-1.24) | 1.00 |
| 211-240 | 222 (0.06) | 167 (0.04) | 1.33 (1.09-1.63) | .005 |
| 241-270 | 185 (0.05) | 162 (0.04) | 1.14 (0.93-1.41) | .22 |
| 271-300 | 199 (0.05) | 171 (0.05) | 1.16 (0.95-1.43) | .15 |
| 301-330 | 196 (0.05) | 155 (0.04) | 1.27 (1.02-1.56) | .03 |
| 331-360 | 183 (0.05) | 176 (0.05) | 1.04 (0.85-1.28) | .71 |
| Ischemic stroke | ||||
| 1-30 | 979 (0.26) | 195 (0.05) | 5.04 (4.32-5.88) | <.001 |
| 31-60 | 307 (0.08) | 170 (0.05) | 1.81 (1.50-2.18) | <.001 |
| 61-90 | 232 (0.06) | 154 (0.04) | 1.51 (1.23-1.85) | <.001 |
| 91-120 | 195 (0.05) | 169 (0.05) | 1.15 (0.94-1.42) | .17 |
| 121-150 | 196 (0.05) | 153 (0.04) | 1.28 (1.04-1.58) | .02 |
| 151-180 | 168 (0.04) | 141 (0.04) | 1.19 (0.95-1.49) | .13 |
| 181-210 | 144 (0.04) | 122 (0.03) | 1.18 (0.93-1.50) | .18 |
| 211-240 | 156 (0.04) | 157 (0.04) | 0.99 (0.80-1.24) | .95 |
| 241-270 | 126 (0.03) | 144 (0.04) | 0.88 (0.69-1.11) | .27 |
| 271-300 | 128 (0.03) | 144 (0.04) | 0.89 (0.70-1.13) | .33 |
| 301-330 | 144 (0.04) | 162 (0.04) | 0.89 (0.71-1.11) | .30 |
| 331-360 | 138 (0.04) | 125 (0.03) | 1.10 (0.87-1.41) | .42 |
| Period before cancer diagnosis, d . | Cancer patients . | Cancer-free controls . | . | . |
|---|---|---|---|---|
| Outcomes, no. (%) . | Outcomes, no. (%) . | OR (95% CI) . | P . | |
| Myocardial infarction | ||||
| 1-30 | 1408 (0.38) | 220 (0.06) | 6.43 (5.57-7.41) | <.001 |
| 31-60 | 340 (0.09) | 201 (0.05) | 1.69 (1.42-2.01) | <.001 |
| 61-90 | 274 (0.07) | 170 (0.05) | 1.61 (1.33-1.95) | <.001 |
| 91-120 | 241 (0.06) | 187 (0.05) | 1.29 (1.07-1.56) | .009 |
| 121-150 | 237 (0.06) | 192 (0.05) | 1.24 (1.02-1.50) | .03 |
| 151-180 | 222 (0.06) | 200 (0.0%) | 1.11 (0.92-1.34) | .28 |
| 181-210 | 170 (0.05) | 170 (0.05) | 1.00 (0.81-1.24) | 1.00 |
| 211-240 | 222 (0.06) | 167 (0.04) | 1.33 (1.09-1.63) | .005 |
| 241-270 | 185 (0.05) | 162 (0.04) | 1.14 (0.93-1.41) | .22 |
| 271-300 | 199 (0.05) | 171 (0.05) | 1.16 (0.95-1.43) | .15 |
| 301-330 | 196 (0.05) | 155 (0.04) | 1.27 (1.02-1.56) | .03 |
| 331-360 | 183 (0.05) | 176 (0.05) | 1.04 (0.85-1.28) | .71 |
| Ischemic stroke | ||||
| 1-30 | 979 (0.26) | 195 (0.05) | 5.04 (4.32-5.88) | <.001 |
| 31-60 | 307 (0.08) | 170 (0.05) | 1.81 (1.50-2.18) | <.001 |
| 61-90 | 232 (0.06) | 154 (0.04) | 1.51 (1.23-1.85) | <.001 |
| 91-120 | 195 (0.05) | 169 (0.05) | 1.15 (0.94-1.42) | .17 |
| 121-150 | 196 (0.05) | 153 (0.04) | 1.28 (1.04-1.58) | .02 |
| 151-180 | 168 (0.04) | 141 (0.04) | 1.19 (0.95-1.49) | .13 |
| 181-210 | 144 (0.04) | 122 (0.03) | 1.18 (0.93-1.50) | .18 |
| 211-240 | 156 (0.04) | 157 (0.04) | 0.99 (0.80-1.24) | .95 |
| 241-270 | 126 (0.03) | 144 (0.04) | 0.88 (0.69-1.11) | .27 |
| 271-300 | 128 (0.03) | 144 (0.04) | 0.89 (0.70-1.13) | .33 |
| 301-330 | 144 (0.04) | 162 (0.04) | 0.89 (0.71-1.11) | .30 |
| 331-360 | 138 (0.04) | 125 (0.03) | 1.10 (0.87-1.41) | .42 |
Stage analyses
Among cancer patients who had an arterial thromboembolic event within 360 days of cancer diagnosis, the cancer stage at diagnosis was stage 0/I in 1871 (28%), stage II in 1065 (16%), stage III in 1044 (16%), stage IV in 1596 (24%), and an unknown stage in 991 (15%). Higher cancer stages at cancer diagnosis were associated with increased odds of an arterial thromboembolic event. For instance, compared with cancer-free controls, cancer patients’ relative odds for an arterial thromboembolic event in the 360 days before cancer diagnosis was 1.32 (95% CI, 1.22-1.43) for stage I cancers, 1.55 (95% CI, 1.40-1.70) for stage II cancers, 2.19 (95% CI, 1.96-2.44) for stage III cancers, and 2.17 (95% CI, 1.99-2.37) for stage IV cancers. These analyses were similar when evaluating myocardial infarction alone and ischemic stroke alone.
Exploratory analysis
When using an expanded definition of arterial thromboembolic events that included systemic artery thromboembolism and acute mesenteric ischemia, in the 360 days before study entry, 7263 (1.94%) cancer patients were diagnosed with an arterial thromboembolic event vs 4215 (1.13%) cancer-free control patients (OR, 1.74; 95% CI, 1.68-1.81; P < .001). This included 491 (0.13%) systemic artery thromboembolism and 304 (0.08%) acute mesenteric ischemia diagnoses among cancer patients and 209 (0.06%) systemic artery thromboembolism and 127 (0.03%) acute mesenteric ischemia diagnoses among cancer-free controls. The risk of the expanded arterial thromboembolic event outcome was highest in the 30 days immediately before the cancer diagnosis (OR, 6.03; 95% CI, 5.45-6.67).
Discussion
In an analysis of >700 000 Medicare beneficiaries, we found that in the 360 days before a cancer diagnosis, the risk of an arterial thromboembolic event was increased nearly 70%. This risk first became increased about 5 months before cancer diagnosis and thereafter progressively rose until peaking in the month before cancer diagnosis, when the risk was increased more than fivefold. The relative risks of myocardial infarction alone and ischemic stroke alone were similar, although myocardial infarction was slightly more common than ischemic stroke. Of the cancer types studied, lung and colorectal cancer were the most likely cancers to be preceded by an arterial thromboembolic event. Meanwhile, among cancer patients who had preceding arterial thromboembolic events, 40% had stages III or IV cancer upon diagnosis.
Few population-based studies have systematically analyzed the association between cancer and preceding arterial thromboembolic events. Among 3680 adults with nondisabling ischemic stroke who participated in a randomized trial of vitamin supplements, the 1-year cancer incidence rate was 1.2%; this was increased 20% compared with the estimated age-adjusted rate in the general population.15 In a prospective Norwegian study of 28 763 patients, incident myocardial infarction was associated with a 46% increased incidence of cancer during follow-up.16 Additionally, in the first 6 months after myocardial infarction, the risk of cancer was increased 120%.16 In a retrospective analysis of 6600 Danish patients with lower limb arterial thrombosis, the incidence rate of subsequent cancer diagnoses was increased 35% compared with expected national rates, and the highest relative risk was in the first 6 months after arterial thrombosis, when it was more than tripled.17 Our study expands on these analyses by including a more heterogeneous patient population, a matched study design whereby cancer patients were matched to cancer-free controls by demographics and comorbidities to lower the risk of confounding, and a substantially larger sample size with resultant increased statistical power, which enabled us to delineate risks by monthly intervals.
Our findings also build on previous studies that evaluated the risk of arterial thromboembolic events after cancer diagnosis. In our prior SEER-Medicare study of 279 719 patients with 8 common cancers, the risk for an arterial thromboembolic event in the 6 months after cancer diagnosis was increased 120% compared with matched controls without cancer.2 Additionally, similar to the current study, risks were higher closer to the time of cancer diagnosis, peaking in the first month afterward, when the hazard ratio for an arterial thromboembolic event was 5.2.2 Meanwhile, nationwide Swedish studies that evaluated >820 000 cancer patients, including cancer types not included in the aforementioned SEER-Medicare study, reported analogous findings, with most cancers conferring a transient increased risk of stroke and coronary heart disease that attenuated or disappeared within 1 year of cancer diagnosis.5,6 By demonstrating that arterial thromboembolism risk increases not only after diagnosis but also several months before, our study suggests that much of the increased risk may be due to the cancer’s intrinsic effects on coagulation, rather than extrinsic factors that only develop after cancer diagnosis, such as cancer treatment effects and antithrombotic interruption for chemotherapy-induced thrombocytopenia. However, antithrombotic interruption for procedures, especially surgical biopsy of a newly discovered mass, might have contributed to the increased arterial thromboembolism risk before cancer diagnosis, particularly in the month or 2 before.
This study has several important limitations. First, it was retrospective and relied on Medicare claims data for arterial thromboembolic event diagnoses and therefore misclassification error is possible. We attempted to minimize this concern by using previously validated diagnostic code algorithms to identify events; however, it remains possible that some diagnoses were incorrect and that other conditions, including cancer metastases, mimicked thromboembolic events. Similarly, by using claims data, we lacked clinical information on lifestyle factors such as smoking, examination and laboratory data, pathophysiological mechanisms, severity of events, and electrocardiographic and imaging findings. We also lacked reliable data on the use of antithrombotic therapy; hence, we were unable to determine whether its interruption for surgical biopsy contributed to arterial thromboembolism risk. Second, because this was a SEER-Medicare study, we restricted our cohort to patients ≥67 years of age, so our results may not apply to younger patients, although in the United States, the estimated median age of patients with cancer is 66 and the estimated mean ages of patients with myocardial infarction and ischemic stroke is 69 and 69, respectively.27-29 Third, because cancer patients were matched to cancer-free controls, only those who survived an arterial thromboembolic event and lived long enough to be diagnosed with cancer were included; therefore, immortal time bias could have affected the association between cancer and preceding arterial thromboembolic events if cancer patients and controls differed in their likelihood to survive events. However, the majority of myocardial infarctions and ischemic strokes are nonfatal and, in practical terms, an association between 2 diseases is most relevant when patients survive the first disease to become at risk for the second.30 Fourth, some arterial thromboembolic events may have led to a diagnosis of cancer through increased medical surveillance. For instance, a chest radiograph performed during myocardial infarction hospitalization might demonstrate a lung mass, which is later biopsied and determined to be cancer. This phenomenon would be most plausible in the month preceding cancer diagnosis and may partly explain the very high odds of thromboembolic events during that month, although some myocardial infarctions probably led to drug-eluting stent placement, which could have delayed cancer diagnosis by several months because of the need for dual antiplatelets to prevent in-stent thrombosis. Fifth, because SEER organizes and releases data by cancer site, we were unable to evaluate all cancer patients registered in SEER. However, we did include 9 common cancer sites, including the 5 most common solid tumor cancers and the most common hematological cancer, which combined account for approximately two-thirds of all cancer in the United States.1 Finally, although we matched on multiple variables, and there were similar rates of unmatched cardiovascular and cancer risk factors between groups, it is possible that residual confounding biased our results. Smoking history, in particular, is not reliably identified through claims data, and any potential imbalances in smoking rates could have confounded the association between cancer and preceding arterial thromboembolic events. However, if present, confounding bias would be expected to produce uniform risk differences between groups over time, whereas in our analysis, risks between groups varied greatly by time.
In summary, the risk of myocardial infarction and ischemic stroke is increased ∼70% in the year before cancer diagnosis. This increased risk begins 5 months before cancer is diagnosed pathologically and peaks in the month before when it is increased fivefold. Risk of arterial thromboembolism preceding cancer diagnosis varies by primary cancer type and seems highest with lung and colorectal cancers. These data suggest that some myocardial infarction and ischemic stroke events may be triggered, or potentially caused by, occult cancer. Future research is needed to identify clinically useful biomarkers for occult cancer in patients with arterial thromboembolic events and to determine the utility of cancer screening strategies in these patients, particularly among those with cancer risk factors or unexplained “cryptogenic” events. In the meantime, we recommend that patients with acute myocardial infarction and ischemic stroke be up-to-date with their age- and gender-appropriate cancer screening, and that their clinicians pay close attention to, and have a low threshold to investigate, any symptoms or signs consistent with occult cancer, such as unexplained anemia or weight loss.
The data used in this analysis include restricted SEER-Medicare claims that, based on the terms of the data use agreement, cannot be shared directly with other investigators. However, investigators can obtain access to these data by application to the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Chen for her copyediting and clerical support, and for assisting in the design and creation of the visual abstract.
This study was supported by research funding grants from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (K23NS091395) (B.B.N.) and National Cancer Institute (P30CA008748) (A.S.R., K.S.P., and L.M.D.), and the Florence Gould Endowment for Discovery in Stroke (B.B.N.). The sponsors had no role in the design and/or execution of this study.
Authorship
Contribution: B.B.N., A.S.R., H.K., K.S.P., and L.M.D. conceived the study and its design; B.B.N. and K.S.P. acquired the data; B.B.N., A.S.R., H.K., C.I., P.M.O., S.T.T., K.S.P., and L.M.D. analyzed and interpreted the data and revised the manuscript for important intellectual content; B.B.N. drafted the manuscript; and B.B.N., A.S.R., and K.S.P. performed the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Babak B. Navi, Weill Cornell Medicine, Department of Neurology, 525 East 68th St, Room F610, New York, NY 10065; e-mail: ban9003@med.cornell.edu.