Key Points
Ravulizumab every 8 weeks is noninferior to eculizumab every 2 weeks across all efficacy end points in C5 inhibitor–naive PNH patients.
Ravulizumab provided immediate, complete, and sustained inhibition of C5 over the entire 8-week dose interval, unlike eculizumab.
Abstract
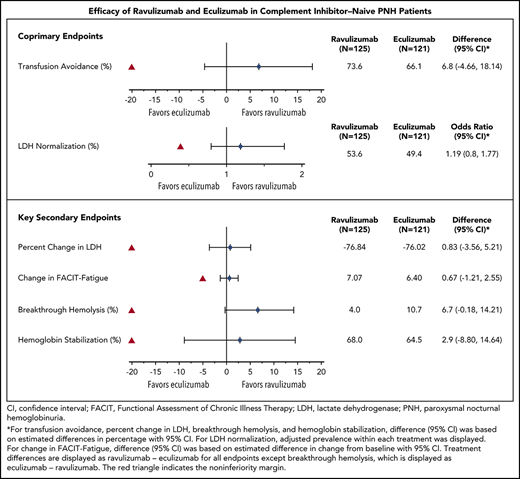
Ravulizumab (ALXN1210), a new complement C5 inhibitor, provides immediate, complete, and sustained C5 inhibition. This phase 3, open-label study assessed the noninferiority of ravulizumab to eculizumab in complement inhibitor–naive adults with paroxysmal nocturnal hemoglobinuria (PNH). Patients with lactate dehydrogenase (LDH) ≥1.5 times the upper limit of normal and at least 1 PNH symptom were randomized 1:1 to receive ravulizumab or eculizumab for 183 days (N = 246). Coprimary efficacy end points were proportion of patients remaining transfusion-free and LDH normalization. Secondary end points were percent change from baseline in LDH, change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue score, proportion of patients with breakthrough hemolysis, stabilized hemoglobin, and change in serum free C5. Ravulizumab was noninferior to eculizumab for both coprimary and all key secondary end points (Pinf < .0001): transfusion avoidance (73.6% vs 66.1%; difference of 6.8% [95% confidence interval (CI), −4.66, 18.14]), LDH normalization (53.6% vs 49.4%; odds ratio, 1.19 [0.80, 1.77]), percent reduction in LDH (−76.8% vs −76.0%; difference [95% CI], −0.83% [−5.21, 3.56]), change in FACIT-Fatigue score (7.07 vs 6.40; difference [95% CI], 0.67 [−1.21, 2.55]), breakthrough hemolysis (4.0% vs 10.7%; difference [95% CI], −6.7% [−14.21, 0.18]), and stabilized hemoglobin (68.0% vs 64.5%; difference [95% CI], 2.9 [−8.80, 14.64]). The safety and tolerability of ravulizumab and eculizumab were similar; no meningococcal infections occurred. In conclusion, ravulizumab given every 8 weeks achieved noninferiority compared with eculizumab given every 2 weeks for all efficacy end points, with a similar safety profile. This trial was registered at www.clinicaltrials.gov as #NCT02946463.
Introduction
Recognition of the pathogenic mechanisms mediated by the complement system led to investigation of complement inhibition as a therapeutic approach for management of paroxysmal nocturnal hemoglobinuria (PNH).1-3 Eculizumab (Soliris; Alexion Pharmaceuticals, Inc, Boston, MA), a humanized monoclonal antibody that blocks terminal complement C5 activation, is the only approved medication for PNH.4-6 Intravenous treatment with eculizumab is associated with sustained improvement in intravascular hemolysis, anemia, transfusion independence, thrombotic events, survival, and quality of life.1-3,7,8 Although the efficacy and safety of eculizumab administered according to the approved every-2-week regimen are well established, the treatment burden associated with this dosing regimen may affect adherence. In addition, 11% to 27% of patients may experience breakthrough hemolysis,9-11 placing patients at risk for thrombotic events and other potentially life-threatening complications associated with intravascular hemolysis.12,13
Ravulizumab (ALXN1210; Alexion Pharmaceuticals, Inc) is a new C5 inhibitor that achieves immediate, complete, and sustained inhibition of complement-mediated hemolysis with an extended dosing interval.14 It exhibits high-affinity binding to C5 and inhibits C5a and C5b formation, thereby preventing immune activation and hemolysis.15,16 Ravulizumab was designed via targeted substitution of 4 amino acids in the complementary binding and neonatal Fc regions of the eculizumab backbone, resulting in augmented endosomal dissociation of C5 and efficient recycling of ravulizumab to the vascular compartment via the neonatal Fc receptor pathway.17 Accordingly, the terminal half-life of ravulizumab is ∼4 times longer than that of eculizumab.14 A >99% reduction in free C5 has been observed as early as the end of the first intravenous infusion of ravulizumab15 ; in phase 1b/2 studies in patients with PNH, ravulizumab elicited immediate and sustained suppression of complement-mediated hemolysis (mean lactate dehydrogenase [LDH] range at baseline, 1027-2142 U/L; mean range at primary end point, 228-306 U/L) throughout dosing intervals up to every 12 weeks.18 In these studies, intravenous ravulizumab dosing that achieved a higher trough exposure was associated with a greater proportion of patients reaching plasma LDH levels within the normal or near-normal range with a lack of breakthrough hemolysis, relative to low trough exposures.18 Subsequent exposure-response analyses informed the weight-based dosing regimen being evaluated in 2 complementary phase 3 studies in PNH patients who are either naive to or receiving stable eculizumab therapy.19 The objective of the current study was to assess the noninferiority of ravulizumab vs eculizumab in adult PNH patients naive to complement inhibitor therapy.
Methods
Trial oversight and study design
The ALXN1210-PNH-301 study (NCT02946463, EudraCT 2016-002025-11, CHAMPION-301), sponsored by Alexion Pharmaceuticals, Inc, is a phase 3, multicenter, randomized, active-controlled, open-label study conducted in 123 centers in 25 countries. The protocol was approved by the institutional review board or independent ethics committee at each participating center, and the study was conducted in accordance with the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines.
The study consisted of a 4-week screening period and a 26-week randomized treatment period to evaluate the efficacy and safety of ravulizumab vs eculizumab, followed by an extension period of up to 2 years, during which all patients receive ravulizumab (supplemental Appendix Section 3; supplemental Figure 1, available on the Blood Web site). Patients were stratified into 6 groups based on transfusion history (0, 1-14, or >14 units of packed red blood cells in the 1 year before the first dose of study drug) and LDH screening level (1.5 to <3 times the upper limit of normal [ULN] or ≥3× ULN). History of major adverse vascular events (MAVEs) was not a component of the randomization stratification criteria. Enrollment of patients without a history of transfusion in the past year was capped at 20%. Hemoglobin levels were evaluated before randomization and within 5 days before study drug initiation; patients were transfused, if necessary, to reach the protocol-specified hemoglobin level.
Patients within each of the 6 groups were randomly assigned in a 1:1 ratio to receive ravulizumab or eculizumab (supplemental Appendix Section 3; supplemental Figure 1). The intravenous ravulizumab group received a loading dose (2400 mg for patients weighing ≥40 to <60 kg, 2700 mg for patients ≥60 kg to <100 kg, and 3000 mg for patients ≥100 kg) on day 1, followed by maintenance doses of ravulizumab (3000 mg for patients ≥40 to <60 kg, 3300 mg for patients ≥60 to <100 kg, and 3600 mg for patients ≥100 kg) on day 15 and every 8 weeks thereafter. Patients assigned to eculizumab received induction doses of 600 mg on days 1, 8, 15, and 22, followed by maintenance dosing of 900 mg on day 29 and every 2 weeks thereafter per the approved PNH dosing regimen.4,5
To reduce the risk of meningococcal infection (Neisseria meningitidis), all patients must have been vaccinated against meningococcal infections within 3 years before, or at the time of, initiating study drug. Patients who initiated study drug treatment <2 weeks after receiving a meningococcal vaccine were required to receive treatment with appropriate prophylactic antibiotics until at least 2 weeks after vaccination. Routine prophylactic antibiotic treatment was otherwise optional.
Patients
The study enrolled patients ≥18 years of age with documented diagnosis of PNH, confirmed by high-sensitivity flow cytometry of red and white blood cells with granulocyte or monocyte clone size of at least 5%, and LDH level ≥1.5× ULN at screening. Within 3 months of screening, ≥1 of the following PNH-related signs or symptoms must have been present: fatigue, hemoglobinuria, abdominal pain, shortness of breath (dyspnea), anemia (ie, hemoglobin level <10 g/dL), or history of MAVEs (including thrombosis), dysphagia, erectile dysfunction, or history of packed red blood cell transfusion because of PNH. Key exclusion criteria included current or previous exposure to a complement inhibitor; weight <40 kg; history of bone marrow transplantation; history of meningococcal or unexplained, recurrent infection; platelet count <30 × 109/L; or absolute neutrophil count <0.5 × 109/L at screening. All patients gave written informed consent. Full eligibility criteria are presented in supplemental Appendix Section 2).
Outcomes
The coprimary end points were: (1) transfusion avoidance (TA), defined as the proportion of patients who remain transfusion-free and do not require a transfusion per protocol-specified guidelines through day 183; and (2) hemolysis as measured by LDH normalization (ULN, 246 U/L) from days 29 through 183. Red blood cell transfusions were administered when patients had a hemoglobin level ≤9 g/dL with anemia-related signs or symptoms of sufficient severity to warrant transfusion or a hemoglobin level ≤7 g/dL regardless of the presence of clinical signs or symptoms.
Key secondary end points included percentage change from baseline to day 183 in LDH and change from baseline to day 183 in quality of life, as assessed via the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale, version 420,21 ; the proportion of patients with breakthrough hemolysis, defined as ≥1 new or worsening sign or symptom of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin <10 g/dL], MAVEs including thrombosis, dysphagia, or erectile dysfunction) in the presence of LDH ≥2× ULN after prior reduction of LDH to <1.5× ULN on treatment, and proportion of patients with stabilized hemoglobin, defined as avoidance of a ≥2 g/dL decrease in hemoglobin level from baseline in the absence of transfusion. Additional secondary end points included time to first occurrence of LDH normalization, total number of packed red blood cell units transfused, change in clinical manifestations of PNH, change from baseline in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 Scale (EORTC QLQ-C30), version 3.0,22 proportion of patients experiencing MAVEs (including thrombosis), and change in free C5 concentrations. Additional details are available in supplemental Appendix Section 2.
Adverse events (AEs) were documented and immunogenicity, reflected by development of antidrug antibodies, was also monitored. A safety review committee performed safety monitoring, and an independent data monitoring committee was in place to monitor meningococcal infections.
Statistical analysis
The planned sample size of ∼214 evaluable patients would provide 80% power to demonstrate noninferiority of ravulizumab to eculizumab at a 1-sided α level of 0.025, a 10% dropout rate, and a noninferiority margin of −20% for the difference in TA rate (minimum of 193 patients required). The sample size estimate based on LDH normalization was smaller than that based on TA (minimum of 142 patients required to provide 80% power). Noninferiority of ravulizumab was based on the coprimary end points and defined as: (1) the lower bound of the 95% confidence interval (CI) for the difference in TA rate between ravulizumab and eculizumab being greater than −20%, and (2) lower bound of the 95% CI for the odds ratio (OR) of ravulizumab vs eculizumab for LDH normalization being greater than an OR of 0.39.
The coprimary end point TA was evaluated as the proportion of patients achieving the end point, computed as a weighted combination of differences between the treatment groups within the 2 randomization stratifications, using Mantel-Haenszel tests. The stratified Newcombe method was used to calculate 95% CIs for TA. ORs and 95% CIs for the coprimary end point of LDH normalization were analyzed using a generalized estimating equation approach with first-order autoregressive correlation structure. The model included LDH normalization as the dependent variable and an indicator variable for treatment, history of transfusion (as a categorical variable based on the stratification factor levels), and baseline LDH level (as a continuous variable).
The key secondary end points were tested in a hierarchical manner if noninferiority was declared for the coprimary end points. If noninferiority was established for all key secondary end points, then superiority was assessed in the following order: breakthrough hemolysis, percentage change in LDH, LDH normalization, change in FACIT-Fatigue score, hemoglobin stabilization, and TA.
To assess the strength of evidence of the study results, post hoc P values were calculated for testing of noninferiority (Pinf) relative to the prespecified noninferiority margins. Additional details are available in supplemental Appendix Section 2.
Baseline LDH was defined as the average of all available assessments before the first infusion of study drug. Baseline was defined as the last available assessment before first study drug infusion for all other parameters.
Efficacy analyses were performed on the full analysis set, which included all patients who received ≥ 1 dose of ravulizumab or eculizumab and had ≥ 1 efficacy assessment after the first infusion. Safety analyses were performed on the safety set, defined as all patients who received ≥ 1 dose of study drug. Pharmacodynamic analyses were performed on all patients who received ≥1 dose of study drug and had evaluable pharmacodynamic data. All analyses were performed using SAS (SAS Institute Inc, Cary, NC), version 9.4, or higher or other validated statistical software.
Results
Patient characteristics
Of 285 patients screened for eligibility, 246 (all of whom received meningococcal vaccination) were randomized to ravulizumab (n = 125) or eculizumab (n = 121); 244 patients completed the 26-week treatment period (ravulizumab, n = 125; eculizumab, n = 119; supplemental Appendix Section 3; supplemental Figure 2). There were no noteworthy differences between treatment groups in demographics or baseline clinical characteristics (Table 1). Overall, 99.2% of patients received all planned infusions of study medication.
Demographics and baseline clinical characteristics
| Characteristic . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . | Total (N = 246) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 65 (52.0) | 69 (57.0) | 134 (54.5) |
| Female | 60 (48.0) | 52 (43.0) | 112 (45.5) |
| Age at first infusion of study drug, mean (SD), y | 44.8 (15.2) | 46.2 (16.2) | 45.5 (15.7) |
| Race, n (%) | |||
| Asian | 72 (57.6) | 57 (47.1) | 129 (52.4) |
| Japanese | 19 (15.2) | 15 (12.4) | 34 (13.8) |
| White | 43 (34.4) | 51 (42.1) | 94 (38.2) |
| Black or African American | 2 (1.6) | 4 (3.3) | 6 (2.4) |
| American Indian or Alaska Native | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Other | 4 (3.2) | 4 (3.3) | 8 (3.3) |
| Not reported | 3 (2.4) | 4 (3.3) | 7 (2.8) |
| Weight, mean (SD), kg | 68.2 (15.6) | 69.2 (14.9) | 68.7 (15.2) |
| Height, mean (SD), cm | 166.3 (9.0) | 166.2 (10.7) | 166.2 (9.8) |
| LDH ratio, n (%) | |||
| 1.5 to <3× ULN* | 18 (14.4) | 16 (13.2) | 34 (13.8) |
| ≥3× ULN | 107 (85.6) | 105 (86.8) | 212 (86.2) |
| Packed RBC units received within 1 y before study entry, randomization strata, n (%) | |||
| 0 U | 23 (18.4) | 21 (17.4) | 44 (17.9) |
| 1-14 U | 79 (63.2) | 78 (64.5) | 157 (63.8) |
| >14 U | 23 (18.4) | 22 (18.2) | 45 (18.3) |
| Age at PNH diagnosis, mean (SD), y | 37.9 (14.9)† | 39.6 (16.7)‡ | 38.7 (15.8)§ |
| Number of years from PNH diagnosis to consent, median (minimum, maximum), y | 3.8 (0, 41)† | 3.9 (0, 34)‡ | 3.9 (0, 41)§ |
| LDH, mean (SD), U/L | 1633.5 (778.8) | 1578.3 (727.1) | 1606.4 (752.7) |
| PNH clone size, mean (SD), % | |||
| Type II RBCs | 12.4 (20.5)|| | 13.7 (17.7)¶ | 13.0 (19.2)# |
| Type III RBCs | 26.3 (17.2)|| | 25.2 (16.9)¶ | 25.8 (17.1)# |
| Total RBCs | 38.4 (23.7) | 38.7 (23.2) | 38.6 (23.4) |
| Granulocytes | 84.2 (21.0) | 85.3 (19.0) | 84.7 (20.0) |
| Monocytes | 86.9 (18.1) | 89.2 (15.2) | 88.0 (16.7) |
| History of major adverse vascular events, n (%) | 17 (13.6) | 25 (20.7) | 42 (17.1) |
| Characteristic . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . | Total (N = 246) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 65 (52.0) | 69 (57.0) | 134 (54.5) |
| Female | 60 (48.0) | 52 (43.0) | 112 (45.5) |
| Age at first infusion of study drug, mean (SD), y | 44.8 (15.2) | 46.2 (16.2) | 45.5 (15.7) |
| Race, n (%) | |||
| Asian | 72 (57.6) | 57 (47.1) | 129 (52.4) |
| Japanese | 19 (15.2) | 15 (12.4) | 34 (13.8) |
| White | 43 (34.4) | 51 (42.1) | 94 (38.2) |
| Black or African American | 2 (1.6) | 4 (3.3) | 6 (2.4) |
| American Indian or Alaska Native | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Other | 4 (3.2) | 4 (3.3) | 8 (3.3) |
| Not reported | 3 (2.4) | 4 (3.3) | 7 (2.8) |
| Weight, mean (SD), kg | 68.2 (15.6) | 69.2 (14.9) | 68.7 (15.2) |
| Height, mean (SD), cm | 166.3 (9.0) | 166.2 (10.7) | 166.2 (9.8) |
| LDH ratio, n (%) | |||
| 1.5 to <3× ULN* | 18 (14.4) | 16 (13.2) | 34 (13.8) |
| ≥3× ULN | 107 (85.6) | 105 (86.8) | 212 (86.2) |
| Packed RBC units received within 1 y before study entry, randomization strata, n (%) | |||
| 0 U | 23 (18.4) | 21 (17.4) | 44 (17.9) |
| 1-14 U | 79 (63.2) | 78 (64.5) | 157 (63.8) |
| >14 U | 23 (18.4) | 22 (18.2) | 45 (18.3) |
| Age at PNH diagnosis, mean (SD), y | 37.9 (14.9)† | 39.6 (16.7)‡ | 38.7 (15.8)§ |
| Number of years from PNH diagnosis to consent, median (minimum, maximum), y | 3.8 (0, 41)† | 3.9 (0, 34)‡ | 3.9 (0, 41)§ |
| LDH, mean (SD), U/L | 1633.5 (778.8) | 1578.3 (727.1) | 1606.4 (752.7) |
| PNH clone size, mean (SD), % | |||
| Type II RBCs | 12.4 (20.5)|| | 13.7 (17.7)¶ | 13.0 (19.2)# |
| Type III RBCs | 26.3 (17.2)|| | 25.2 (16.9)¶ | 25.8 (17.1)# |
| Total RBCs | 38.4 (23.7) | 38.7 (23.2) | 38.6 (23.4) |
| Granulocytes | 84.2 (21.0) | 85.3 (19.0) | 84.7 (20.0) |
| Monocytes | 86.9 (18.1) | 89.2 (15.2) | 88.0 (16.7) |
| History of major adverse vascular events, n (%) | 17 (13.6) | 25 (20.7) | 42 (17.1) |
PNH, paroxysmal nocturnal hemoglobinuria; RBC, red blood cell; SD, standard deviation.
The ULN for LDH is 246 U/L.
n = 123.
n = 118.
n = 241.
n = 124.
n = 120.
n = 244.
Coprimary end points
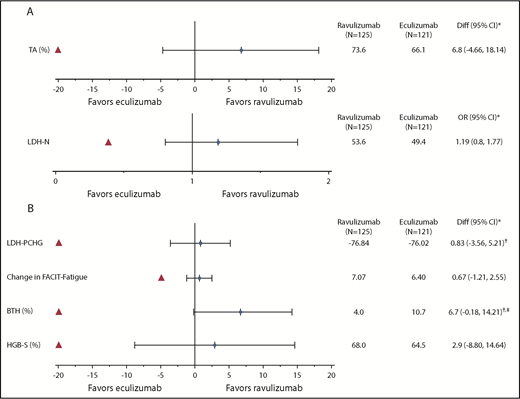
Ravulizumab met the objective of noninferiority compared with eculizumab on both coprimary end points (Figure 1A; Table 2), and point estimates for both coprimary end points favored ravulizumab. Ninety-two of 125 patients (73.6%) receiving ravulizumab and 80 of 121 patients (66.1%) receiving eculizumab avoided transfusion, with a between-group difference of 6.8% (95% CI, −4.66, 18.14; Pinf < .0001). The lower bound of the 95% CI was greater than the protocol-specified noninferiority margin of −20%. The adjusted prevalence of LDH normalization was 53.6% for the ravulizumab group and 49.4% for the eculizumab group; the adjusted OR for comparison of ravulizumab vs eculizumab was 1.19 (95% CI, 0.80, 1.77; Pinf < .0001). The lower bound of the 95% CI was greater than the protocol-specified noninferiority margin of 0.39.
Treatment effect for the coprimary and key secondary efficacy end points. (A) Treatment difference is estimated for ravulizumab-eculizumab. For the TA end point, treatment differences (Diff) (95% CI) are based on estimated differences in percent with 95% CI. For the LDH normalization (LDH-N) end point, adjusted prevalence within each treatment is displayed. *Red triangle indicates the noninferiority margin. (B) For key secondary end points LDH-PCHG (percent change), breakthrough hemolysis (BTH), and hemoglobin stabilization (HGB-S), Diff (95% CI) is based on estimated differences in percent with 95% CI. For FACIT-Fatigue, Diff (95% CI) is based on estimated differences in change from baseline with 95% CI. *Red triangle indicates the noninferiority margin. †Treatment difference is estimated for ravulizumab-eculizumab except for LDH-PCHG and BTH, where treatment difference is based on eculizumab-ravulizumab. ‡P < .06 for the lower bound of the 95% CI.
Treatment effect for the coprimary and key secondary efficacy end points. (A) Treatment difference is estimated for ravulizumab-eculizumab. For the TA end point, treatment differences (Diff) (95% CI) are based on estimated differences in percent with 95% CI. For the LDH normalization (LDH-N) end point, adjusted prevalence within each treatment is displayed. *Red triangle indicates the noninferiority margin. (B) For key secondary end points LDH-PCHG (percent change), breakthrough hemolysis (BTH), and hemoglobin stabilization (HGB-S), Diff (95% CI) is based on estimated differences in percent with 95% CI. For FACIT-Fatigue, Diff (95% CI) is based on estimated differences in change from baseline with 95% CI. *Red triangle indicates the noninferiority margin. †Treatment difference is estimated for ravulizumab-eculizumab except for LDH-PCHG and BTH, where treatment difference is based on eculizumab-ravulizumab. ‡P < .06 for the lower bound of the 95% CI.
Coprimary and key secondary efficacy outcomes at day 183
| . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . | Statistic for comparison . | Treatment effect . | Noninferiority margin . | Conclusion . |
|---|---|---|---|---|---|---|
| Coprimary end points | ||||||
| Transfusion avoidance rate, % (95% CI) | 73.6 (65.87, 81.33) | 66.1 (57.68, 74.55) | Difference in rate | 6.8 (−4.66, 18.14) | −20% | Noninferior |
| LDH normalization, % (95% CI) | 53.6 (45.9, 61.2) | 49.4 (41.7, 57.0) | OR | 1.19 (0.80, 1.77) | 0.39 | Noninferior |
| Key secondary efficacy end points | ||||||
| LDH, least squares mean % change (95% CI) | −76.84 (−79.96, −73.73) | −76.02 (−79.20, −72.83) | Difference in % change from baseline | −0.83 (−5.21, 3.56) | 20% | Noninferior |
| FACIT-Fatigue score, least squares mean change (95% CI) | 7.07 (5.55, 8.60) | 6.40 (4.85, 7.96) | Difference in change from baseline | 0.67 (−1.21, 2.55) | −5.0 | Noninferior |
| Breakthrough hemolysis rate, % (95% CI) | 4.0 (0.56, 7.44) | 10.7 (5.23, 16.26) | Difference in rate | −6.7 (−14.21, 0.18) | 20% | Noninferior |
| Hemoglobin stabilization rate, % (95% CI) | 68.0 (59.82, 76.18) | 64.5 (55.93, 72.99) | Difference in rate | 2.9 (−8.80, 14.64) | −20% | Noninferior |
| . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . | Statistic for comparison . | Treatment effect . | Noninferiority margin . | Conclusion . |
|---|---|---|---|---|---|---|
| Coprimary end points | ||||||
| Transfusion avoidance rate, % (95% CI) | 73.6 (65.87, 81.33) | 66.1 (57.68, 74.55) | Difference in rate | 6.8 (−4.66, 18.14) | −20% | Noninferior |
| LDH normalization, % (95% CI) | 53.6 (45.9, 61.2) | 49.4 (41.7, 57.0) | OR | 1.19 (0.80, 1.77) | 0.39 | Noninferior |
| Key secondary efficacy end points | ||||||
| LDH, least squares mean % change (95% CI) | −76.84 (−79.96, −73.73) | −76.02 (−79.20, −72.83) | Difference in % change from baseline | −0.83 (−5.21, 3.56) | 20% | Noninferior |
| FACIT-Fatigue score, least squares mean change (95% CI) | 7.07 (5.55, 8.60) | 6.40 (4.85, 7.96) | Difference in change from baseline | 0.67 (−1.21, 2.55) | −5.0 | Noninferior |
| Breakthrough hemolysis rate, % (95% CI) | 4.0 (0.56, 7.44) | 10.7 (5.23, 16.26) | Difference in rate | −6.7 (−14.21, 0.18) | 20% | Noninferior |
| Hemoglobin stabilization rate, % (95% CI) | 68.0 (59.82, 76.18) | 64.5 (55.93, 72.99) | Difference in rate | 2.9 (−8.80, 14.64) | −20% | Noninferior |
For the transfusion avoidance end point, treatment differences (95% CIs) are based on estimated differences in percent with 95% CI. For the LDH-N end point, the adjusted prevalence within each treatment is displayed. Testing of the noninferiority hypothesis is assessed by comparing the bolded limit of the 95% CI to the noninferiority margin.
Key secondary end points
Ravulizumab was noninferior to eculizumab on the 4 key secondary end points (Figure 1B; Table 2), with all point estimates consistently favoring ravulizumab. The between-group difference in least-squares mean percentage change in LDH levels was −0.83% (95% CI, −5.21, 3.56; Pinf < .0001); least-squares mean difference in change in FACIT-Fatigue score was 0.67 (95% CI, −1.21, 2.55; Pinf < .0001). Proportions of patients with breakthrough hemolysis were 4.0% (5 of 125 patients had 1 event each) in the ravulizumab group vs 10.7% (13 of 121 patients had a total of 15 events) in the eculizumab group (difference, −6.7% [95% CI, −14.21, 0.18]; Pinf < .0001). Because noninferiority was achieved for all 4 key secondary end points, hierarchal superiority testing was performed for breakthrough hemolysis, with a resulting P < .06. Of the 5 breakthrough hemolysis events in the ravulizumab group, 4 were associated with infections; the cause of the other was undetermined (ie, unrelated to a known complement amplifying condition/infection or inadequate pharmacodynamic response). None of these events was associated with inadequate terminal complement inhibition (free C5 levels ≥0.5 μg/mL). Of the 15 breakthrough hemolysis events in the eculizumab group, 7 were associated with inadequate terminal complement inhibition, 4 were associated with infections, and 4 had no determined cause. The proportion of patients with stabilized hemoglobin was 68.0% (85 of 125) with ravulizumab vs 64.5% (78 of 121) with eculizumab (difference, 2.9% [95% CI, −8.80, 14.64; Pinf < .0001]).
Additional secondary end points
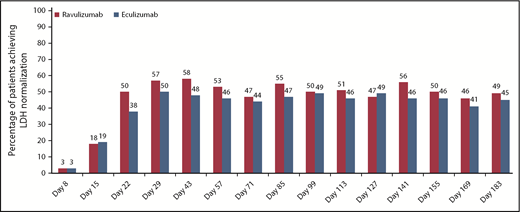
Mean LDH levels decreased and normalized rapidly after initiation of study drug, and LDH normalization was sustained through the 26-week treatment period. Median (95% CI) time to first occurrence of LDH normalization was 5 days shorter in the ravulizumab group (24 [22, 29] days vs 29 [24, 43] days). A greater percentage of patients receiving ravulizumab achieved LDH normalization over the 26-week treatment period (Figure 2). Data regarding packed red blood cell transfusions, incidence of MAVEs, and clinical manifestations of PNH are summarized in Table 3. The mean (SD) total number of packed red blood cell units transfused during the treatment period was comparable in the ravulizumab (4.8 [5.1]) and eculizumab (5.6 [5.9]) groups. Three patients experienced MAVEs, 2 in the ravulizumab group and 1 in the eculizumab group. Patients in both treatment groups reported improvements from baseline in clinical manifestations of PNH (Table 3).
Proportion of patients achieving LDH-N over time in the ravulizumab and eculizumab treatment groups. LDH-N is defined as proportion of patients who achieved LDH level ≤1× ULN (246 U/L).
Proportion of patients achieving LDH-N over time in the ravulizumab and eculizumab treatment groups. LDH-N is defined as proportion of patients who achieved LDH level ≤1× ULN (246 U/L).
Other secondary efficacy outcomes at day 183
| . | Ravulizumab (N = 125) . | Eculizumab (N = 121)* . | ||
|---|---|---|---|---|
| Patients who received packed RBC transfusions, n (%) | 32 (25.6) | 40 (33.1) | ||
| Total number of packed RBC units transfused, mean (SD) | 4.8 (5.1) | 5.6 (5.9) | ||
| Patients with major adverse vascular events, n (%) | 2 (1.6)† | 1 (0.8)‡ | ||
| Clinical manifestations of paroxysmal nocturnal hemoglobinuria, n (%) | Baseline | Day 183 | Baseline | Day 183 |
| Fatigue | 80 (64.0) | 36 (28.8) | 76 (63.9) | 36 (30.3) |
| Abdominal pain | 17 (13.6) | 6 (4.8) | 15 (12.6) | 6 (5.0) |
| Dyspnea | 42 (33.6) | 18 (14.4) | 38 (31.9) | 17 (14.3) |
| Dysphagia | 13 (10.4) | 3 (2.4) | 16 (13.4) | 1 (0.8) |
| Chest pain | 5 (4.0) | 3 (2.4) | 17 (14.3) | 7 (5.9) |
| Hemoglobinuria§ | 71 (56.8) | 13 (10.4) | 56 (47.5) | 11 (9.3) |
| Erectile dysfunction|| | 16 (12.8) | 10 (8.0) | 21 (17.6) | 5 (4.2) |
| . | Ravulizumab (N = 125) . | Eculizumab (N = 121)* . | ||
|---|---|---|---|---|
| Patients who received packed RBC transfusions, n (%) | 32 (25.6) | 40 (33.1) | ||
| Total number of packed RBC units transfused, mean (SD) | 4.8 (5.1) | 5.6 (5.9) | ||
| Patients with major adverse vascular events, n (%) | 2 (1.6)† | 1 (0.8)‡ | ||
| Clinical manifestations of paroxysmal nocturnal hemoglobinuria, n (%) | Baseline | Day 183 | Baseline | Day 183 |
| Fatigue | 80 (64.0) | 36 (28.8) | 76 (63.9) | 36 (30.3) |
| Abdominal pain | 17 (13.6) | 6 (4.8) | 15 (12.6) | 6 (5.0) |
| Dyspnea | 42 (33.6) | 18 (14.4) | 38 (31.9) | 17 (14.3) |
| Dysphagia | 13 (10.4) | 3 (2.4) | 16 (13.4) | 1 (0.8) |
| Chest pain | 5 (4.0) | 3 (2.4) | 17 (14.3) | 7 (5.9) |
| Hemoglobinuria§ | 71 (56.8) | 13 (10.4) | 56 (47.5) | 11 (9.3) |
| Erectile dysfunction|| | 16 (12.8) | 10 (8.0) | 21 (17.6) | 5 (4.2) |
n = 119 patients were included in the analysis of clinical manifestations of PNH.
One patient, who was taking concomitant oral contraceptive medication, experienced an event of lower leg deep vein thrombosis. The other patient had history of lower leg pain and edema and was taking an oral anticoagulant, which was discontinued after initiation of study drug.
This event of mesenteric venous thrombosis with concurrent neutropenic colitis occurred in a patient who had history of aplastic anemia.
n = 118 patients with evaluable data in the eculizumab group.
n = 65 male patients in the ravulizumab group and n = 68 male patients in the eculizumab group.
Improvements in EORTC QLQ-C30 global health status/quality of life assessment scores were also similar in the ravulizumab and eculizumab treatment groups (supplemental Appendix Section 4; supplemental Table 1). A higher percentage of patients in the ravulizumab group experienced a ≥ 10-point improvement in the EORTC QLQ-C30 global health status/quality of life score (difference [95% CI], 4.8 [−7.7, 17.1]), physical functioning score (difference [95% CI], 3.7 [−8.7, 16.0]), and fatigue score (difference [95% CI], 9.1 [−2.5, 20.5]). Similarly, a higher percentage of patients in the ravulizumab group experienced a ≥3-point improvement in FACIT-Fatigue score (61.6%) vs the eculizumab group (58.7%; difference [95% CI], 2.2 [−9.9, 14.3]).
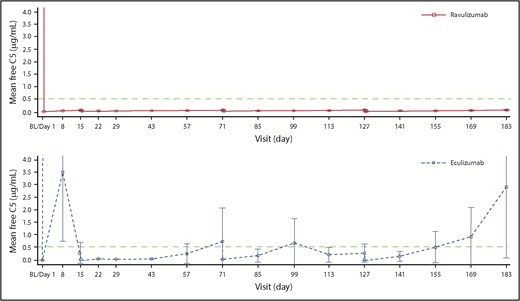
Ravulizumab achieved complete terminal complement inhibition (defined as serum free C5 <0.5 μg/mL) by the end of the first infusion, which was sustained throughout the 183-day treatment period in all patients. This threshold was not consistently met in patients receiving eculizumab (Figure 3).
Mean (95% CI) free C5 concentrations in the ravulizumab and eculizumab groups over time. Free C5 levels were assessed using a Gyros-based fluorescence assay in patients who received ravulizumab and an electrochemiluminescent immunoassay in patients who received eculizumab; 3 patients in the ravulizumab group and 8 in the eculizumab group had day 1 samples excluded because the samples were considered biologically implausible. Free C5 levels <0.5 µg/mL are associated with complete inhibition of C5 activity. Data from days 1, 15, 71, and 127 are from predose and end of infusion for both treatment groups, whereas at days 8, 22, 29, 43, 57, 85, 99, 113, 141, 155, and 169, the data are from any time for the ravulizumab group and predose for the eculizumab group; and at day 183, data are from end of the randomized treatment period for both treatment groups. BL, baseline (the last nonmissing assessment value before first dose of study drug).
Mean (95% CI) free C5 concentrations in the ravulizumab and eculizumab groups over time. Free C5 levels were assessed using a Gyros-based fluorescence assay in patients who received ravulizumab and an electrochemiluminescent immunoassay in patients who received eculizumab; 3 patients in the ravulizumab group and 8 in the eculizumab group had day 1 samples excluded because the samples were considered biologically implausible. Free C5 levels <0.5 µg/mL are associated with complete inhibition of C5 activity. Data from days 1, 15, 71, and 127 are from predose and end of infusion for both treatment groups, whereas at days 8, 22, 29, 43, 57, 85, 99, 113, 141, 155, and 169, the data are from any time for the ravulizumab group and predose for the eculizumab group; and at day 183, data are from end of the randomized treatment period for both treatment groups. BL, baseline (the last nonmissing assessment value before first dose of study drug).
Safety
Ravulizumab and eculizumab were well tolerated in this study. AEs are summarized in Table 4. The most frequently reported AE was headache (36.0% and 33.1% in the ravulizumab and eculizumab groups, respectively). Twenty patients experienced serious AEs (11 ravulizumab and 9 eculizumab patients); pyrexia was the only serious AE reported in >1 patient (1 ravulizumab patient and 2 eculizumab patients). No cases of meningococcal infections, Aspergillus infections, or sepsis were reported. Other serious infections occurred in 2 patients (1.6%) in the ravulizumab group and 4 (3.3%) in the eculizumab group. Serious infections observed in patients treated with ravulizumab included leptospirosis and systemic infection (causative agents not identified); serious infections observed in patients treated with eculizumab included limb abscess, cellulitis, infection, pneumonia, and viral upper respiratory tract infection (causative agents not identified). There were no discontinuations of ravulizumab and 2 discontinuations of eculizumab during the randomized treatment period, 1 due to a physician’s decision and 1 patient withdrew consent. Immunogenicity was low with 1 treatment-emergent antidrug antibody–positive sample in each treatment arm. Antibody titers were low (≤1) and not neutralizing, with no apparent effects on pharmacokinetics/pharmacodynamics or safety.
Adverse events
| Variable . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . |
|---|---|---|
| Patients with AEs, n (%) | 110 (88.0) | 105 (86.8) |
| Most common AEs (≥5% of patients in either treatment group), n (%) | ||
| Headache | 45 (36.0) | 40 (33.1) |
| Nasopharyngitis | 11 (8.8) | 18 (14.9) |
| Nausea | 11 (8.8) | 10 (8.3) |
| Upper respiratory tract infection | 13 (10.4) | 7 (5.8) |
| Pyrexia | 6 (4.8) | 13 (10.7) |
| Viral upper respiratory tract infection | 9 (7.2) | 10 (8.3) |
| Arthralgia | 8 (6.4) | 8 (6.6) |
| Dizziness | 9 (7.2) | 7 (5.8) |
| Pain in extremity | 9 (7.2) | 7 (5.8) |
| Diarrhea | 10 (8.0) | 5 (4.1) |
| Myalgia | 7 (5.6) | 9 (7.4) |
| Abdominal pain | 7 (5.6) | 7 (5.8) |
| Oropharyngeal pain | 8 (6.4) | 6 (5.0) |
| Back pain | 7 (5.6) | 6 (5.0) |
| Cough | 4 (3.2) | 8 (6.6) |
| Hypokalemia | 6 (4.8) | 6 (5.0) |
| Dyspepsia | 4 (3.2) | 6 (5.0) |
| Insomnia | 2 (1.6) | 6 (5.0) |
| Patients with serious AEs, n (%)* | 11 (8.8) | 9 (7.4) |
| Meningococcal infections, n (%) | 0 | 0 |
| Death, n (%) | 0 | 1 (0.8)† |
| Patients with AEs leading to withdrawal of study drug, n (%) | 0 | 1 (0.8)† |
| Patients with serious AEs leading to withdrawal of study drug, n (%) | 0 | 1 (0.8) |
| Variable . | Ravulizumab (N = 125) . | Eculizumab (N = 121) . |
|---|---|---|
| Patients with AEs, n (%) | 110 (88.0) | 105 (86.8) |
| Most common AEs (≥5% of patients in either treatment group), n (%) | ||
| Headache | 45 (36.0) | 40 (33.1) |
| Nasopharyngitis | 11 (8.8) | 18 (14.9) |
| Nausea | 11 (8.8) | 10 (8.3) |
| Upper respiratory tract infection | 13 (10.4) | 7 (5.8) |
| Pyrexia | 6 (4.8) | 13 (10.7) |
| Viral upper respiratory tract infection | 9 (7.2) | 10 (8.3) |
| Arthralgia | 8 (6.4) | 8 (6.6) |
| Dizziness | 9 (7.2) | 7 (5.8) |
| Pain in extremity | 9 (7.2) | 7 (5.8) |
| Diarrhea | 10 (8.0) | 5 (4.1) |
| Myalgia | 7 (5.6) | 9 (7.4) |
| Abdominal pain | 7 (5.6) | 7 (5.8) |
| Oropharyngeal pain | 8 (6.4) | 6 (5.0) |
| Back pain | 7 (5.6) | 6 (5.0) |
| Cough | 4 (3.2) | 8 (6.6) |
| Hypokalemia | 6 (4.8) | 6 (5.0) |
| Dyspepsia | 4 (3.2) | 6 (5.0) |
| Insomnia | 2 (1.6) | 6 (5.0) |
| Patients with serious AEs, n (%)* | 11 (8.8) | 9 (7.4) |
| Meningococcal infections, n (%) | 0 | 0 |
| Death, n (%) | 0 | 1 (0.8)† |
| Patients with AEs leading to withdrawal of study drug, n (%) | 0 | 1 (0.8)† |
| Patients with serious AEs leading to withdrawal of study drug, n (%) | 0 | 1 (0.8) |
Serious AEs in the ravulizumab group included: anemia, aplastic anemia, neutropenia, thrombocytopenia, left ventricular failure, myocardial ischemia, pyrexia, leptospirosis, systemic infection, laceration, uterine leiomyoma, renal colic, and deep vein thrombosis (n = 1 patient each). Serious AEs in the eculizumab group included: pyrexia (n = 2 patients), ileus, neutropenic colitis, limb abscess, cellulitis, infection, pneumonia, viral upper respiratory tract infection, adenocarcinoma of colon, lung adenocarcinoma, and paroxysmal nocturnal hemoglobinuria (n = 1 patient each).
One patient in the eculizumab arm died of lung cancer (unrelated to treatment) during the extension phase of the study.
Discussion
In this largest controlled study of patients with PNH who were naive to complement inhibitor therapy, treatment with ravulizumab when administered every 8 weeks met both coprimary end points (avoidance of red blood cell transfusion and normalization of LDH) and all key secondary end points, showing noninferiority to treatment every 2 weeks with eculizumab (Pinf < .0001 for all end points). For all efficacy end points, the large differences between the boundaries of the CIs and noninferiority margins establish the strength of evidence of the study results. The robust results observed in this study across a broad selection of clinically relevant end points, despite the challenges of using a highly efficacious comparator, reflect treatment of a large patient population, high compliance with respect to study execution, and statistical rigor.
Transfusion requirements and elevated hemolysis (LDH levels ≥1.5× ULN) are both important measures of PNH disease severity1,3,12 and are associated with negative outcomes in patients with PNH.12,23 Transfusion is both a supportive modality and an important measure of disease activity before and during treatment. Nevertheless, not all patients with PNH experience hemolytic anemia that is severe enough to warrant red blood cell transfusions.12,23 In addition, reports from the International PNH Registry13,24 and others12,23 have identified elevated hemolysis, or more specifically, increases in LDH, as a prognostic indicator in PNH. Indeed, presence of ≥1 reported PNH-related symptom in addition to hemolysis confers particularly high risk of thrombosis-related complications.12,25 Complement inhibition mitigates these poor outcomes26 through the inhibition of intravascular hemolysis, stabilization of hemoglobin levels, and reduced need for transfusions.
Between 11% and 27% of eculizumab-treated patients experience breakthrough hemolysis,9-11 which may lead to a return of the symptoms and complications of PNH.6 In this study, 5 ravulizumab-treated patients experienced breakthrough hemolysis compared with 13 patients treated with eculizumab (P < .06) at the approved dose; higher doses than the approved dose of eculizumab were not permitted in this study. This outcome is clinically relevant for patients with PNH, some of whom do not achieve complete disease control with the current standard of care, labeled-dose eculizumab (900 mg every 2 weeks as maintenance).9-11 Therefore, the results of this study demonstrating a trend favoring ravulizumab for all 6 end points are most likely driven by the sustained inhibition of C5 associated with ravulizumab. One patient from the Republic of Korea had a heterozygous missense mutation (c.2654G>A in exon 21) of the C5 gene that confers low response to eculizumab27 and, as expected, did not experience a clinical response to ravulizumab.
The safety profile of ravulizumab in patients with PNH who were naive to complement inhibitor therapy was comparable to that of eculizumab.1,2,10 As observed in previous studies with eculizumab,1,2,10 the most frequently reported AE was headache. The serious infections noted in this study resolved without sequelae. No meningococcal infections occurred during the study. Nonetheless, because terminal complement plays a major role in prevention of meningococcal disease6,28 and patients treated with eculizumab are at increased risk for meningococcal infection, it is expected that this would also be the case for ravulizumab.4,5,29,30
From a patient and health care perspective, a fourfold longer dosing interval of ravulizumab vs eculizumab may reduce treatment burden and health care resource utilization and may expand access to patients who are unable to comply with the every-2-week dosing of eculizumab.
In conclusion, ravulizumab given every 8 weeks achieved noninferiority compared with eculizumab given every 2 weeks for both coprimary end points and all 4 key secondary efficacy end points by providing immediate, complete, and sustained inhibition of terminal complement with a safety profile similar to that of eculizumab.
Portions of this work were presented at the 23rd Congress of the European Hematology Association, Stockholm, Sweden, 14-17 June 2018.
Qualified academic investigators may request participant-level, deidentified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to this study. Further details regarding data availability, instructions for requesting information, and our data disclosure policy will be available on the Alexion Web site (http://alexion.com/research-development).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Peloton Advantage, LLC, which provided editorial and medical writing support by Michael D. Morren, with funding from Alexion Pharmaceuticals, Inc. The authors thank Rodrigo Pavani and Masayo Ogawa of Alexion Pharmaceuticals, Inc, for their contribution to the implementation of the study. Statistical analysis, pharmacokinetic/pharmacodynamic assessments, and editorial review were provided by Andrew I. Damokosh and Stephan Ortiz of Alexion Pharmaceuticals, Inc. Editorial review was also provided by Kenneth Pomerantz of Alexion Pharmaceuticals, Inc. The authors thank the investigators of ALXN1210-PNH-301, who are listed in supplemental Appendix Section 1. The sponsor and investigators also thank the patients and their families for their participation in and support for this clinical study.
This study was supported by Alexion Pharmaceuticals, Inc.
Authorship
Contribution: J.W.L. developed the protocol, recruited patients, and collected data, analyzed and interpreted the data, contributed to the manuscript, and approved the final version; F.S.d.F., L.W.L.L., V. Pessoa, S.G., W.F., and V. Ptushkin contributed to the manuscript and approved the final version; S.T.R., L.V., L.S., R.A., and R.P. developed the protocol, analyzed and interpreted the data, contributed to the manuscript, and approved the final version; and H.S. and A.H. developed the protocol, recruited patients and collected data, analyzed and interpreted the data, contributed to the manuscript, and approved the final version.
Conflict-of-interest disclosure: J.W.L. has received honoraria, consulting fees, and research support (to Seoul St. Mary’s Hospital) from Alexion Pharmaceuticals, Inc. F.S.d.F. has received honoraria and research support (to St. Louis Hospital) from Alexion Pharmaceuticals, Inc. V. Pessoa and S.G. have received research support from Alexion Pharmaceuticals, Inc. W.F. has received honoraria from Alexion Pharmaceuticals, Inc, and Novartis. V. Ptushkin has received honoraria from Alexion Pharmaceuticals, Inc. S.T.R., L.V., L.S., R.A., and R.P. are employees and stockholders of Alexion Pharmaceuticals, Inc. H.S. has received honoraria and research support (all to University of Ulm) from Alexion Pharmaceuticals, Inc. A.H. has received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. L.W.L.L. declares no competing financial interests.
A complete list of ALXN1210-PNH-301 study investigators appears in the supplemental appendix.
Correspondence: Jong Wook Lee, Department of Hematology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Republic of Korea; e-mail: jwlee@catholic.ac.kr.