In this issue of Blood, Lee et al1 and Kulasekararaj et al2 report the results of 2 phase 3, open-label, multicenter trials evaluating the efficacy and safety of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria (PNH).
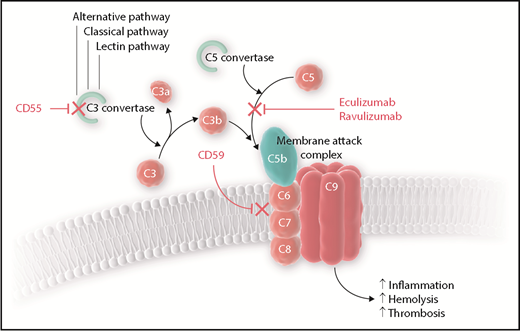
Complement regulation and role of eculizumab and ravulizumab. The alternative, lectin, and classical pathways of complement converge at C3 activation at which point CD55 typically exhibits an inhibitory effect on the formation of the C3 convertase to prevent the formation of C3b. C5 is cleaved by the C5 convertase to C5a and C5b, and then C5b may join with C3b and other complement proteins to form the membrane attack complex (MAC). CD59 inhibits the formation of the MAC. Without CD55 and CD59, complement-mediated cellular destruction leads to increased inflammation, hemolysis, and thrombosis. Both eculizumab and ravulizumab inhibit terminal complement activation by binding to C5, which prevents the formation of C5b and therefore the MAC.
Complement regulation and role of eculizumab and ravulizumab. The alternative, lectin, and classical pathways of complement converge at C3 activation at which point CD55 typically exhibits an inhibitory effect on the formation of the C3 convertase to prevent the formation of C3b. C5 is cleaved by the C5 convertase to C5a and C5b, and then C5b may join with C3b and other complement proteins to form the membrane attack complex (MAC). CD59 inhibits the formation of the MAC. Without CD55 and CD59, complement-mediated cellular destruction leads to increased inflammation, hemolysis, and thrombosis. Both eculizumab and ravulizumab inhibit terminal complement activation by binding to C5, which prevents the formation of C5b and therefore the MAC.
PNH, characterized by cytopenias and thrombosis, is a clonal hematopoietic stem cell disorder in which a mutation in the PIGA gene on the X-chromosome leads to loss of the glycosylphosphatidylinositol (GPI) anchor on the surface of circulating blood cells.3 Normally, red blood cells are protected from complement-mediated hemolysis by the GPI-anchored proteins CD55 and CD59. The lack of GPI on the surface makes these cells susceptible to complement-mediated destruction. For more than a decade, eculizumab has been the only US Food and Drug Administration (FDA)–approved complement inhibitor for the treatment of PNH.4,5 Eculizumab binds to terminal complement C5, thereby preventing the formation of the membrane attack complex and reducing thrombosis, hemolysis, and inflammation (see figure). Maintenance dosing of eculizumab requires that patients be treated every 2 weeks, and although the drug is effective in most patients, some may experience breakthrough hemolysis and fatigue in the days leading up to their next infusion. Ravulizumab is a new complement inhibitor that also binds to C5 but has a much longer terminal half-life compared with eculizumab, which allows a greater interval between infusions.6 Two phase 1b/2 multicenter open-label trials were recently published detailing the efficacy and safety of multiple dose levels and regimens of ravulizumab in PNH patients who had not previously received complement inhibitor therapy.7 The current parallel randomized controlled studies compare ravulizumab to eculizumab for the treatment of PNH in patients who were either complement inhibitor therapy naive (the 301 study; NCT02946463: ALXN1210 Versus Eculizumab in Complement Inhibitor Treatment-Naïve Adult Patients With Paroxysmal Nocturnal Hemoglobinuria [PNH]) or complement inhibitor therapy experienced (the 302 study; NCT03056040: ALXN1210 Versus Eculizumab in Adult Patients With Paroxysmal Nocturnal Hemoglobinuria [PNH] Currently Treated With Eculizumab).
In the 301 study, PNH patients naive to complement inhibition were randomly assigned to ravulizumab (n = 125) or eculizumab (n = 121). Patients were stratified on the basis of transfusion history during the previous year as well as lactate dehydrogenase (LDH) level. Patients assigned to eculizumab underwent induction therapy with 600 mg intravenously on days 1, 8, 15, and 22 followed by 900 mg intravenously on day 29 and then once every 2 weeks. Patients assigned to ravulizumab received a weight-based loading dose intravenously on day 1 followed by a maintenance dose intravenously on day 15 and then once every 8 weeks. All patients received vaccination against Neisseria meningitidis. Coprimary end points were transfusion avoidance and hemolysis measured by LDH. There were also several key secondary end points that included a standardized assessment of fatigue as well as major adverse vascular events that included thrombosis. Ravulizumab met the prespecified noninferiority threshold with 73.6% of study participants avoiding transfusion whereas 66.1% of those receiving eculizumab avoided transfusion. LDH normalization was 53.6% in the ravulizumab arm and 49.4% in the eculizumab arm, which also met prespecified noninferiority criteria. In addition, noninferiority was also achieved for all 4 secondary end points: change in LDH, change in fatigue, breakthrough hemolysis, and hemoglobin stabilization rate.
The most frequently reported adverse event in the 301 study was headache, with similar rates across both treatment arms (ravulizumab 36% and eculizumab 33.1%). Importantly, there were no cases of meningococcal infections, although one patient receiving ravulizumab therapy developed leptospirosis and another developed a serious systemic infection with no known cause.
In the 302 study, patients with PNH who previously received complement therapy with eculizumab were randomly assigned to either continue eculizumab maintenance dosing once every 2 weeks (n = 98) or switch to ravulizumab with a loading dose on day 1 followed by maintenance doses on day 15 and then once every 8 weeks for 26 weeks (n = 97). After the initial 26 weeks of therapy, all patients received ravulizumab for 2 years as part of an extension study. Ravulizumab met the prespecified noninferiority criteria for the primary outcome of percentage change in LDH as well as noninferiority criteria for the same key secondary end points as in the 301 study. As in the 301 study, the most common adverse event in the 302 study was headache (26.8% in the ravulizumab arm and 17.3% in the eculizumab arm), and none of the patients developed meningococcal infection.
Because ravulizumab has a terminal half-life of approximately 32 days, it is not surprising that study participants who received it experienced fewer breakthrough hemolysis events. The 8-week maintenance dosing schedule is likely to be well received by patients, although issues of cost and equitable patient access will need to be addressed. Currently, the average wholesale acquisition cost of eculizumab is around $19 500 US dollars for each maintenance dose and the cost of ravulizumab has yet to be announced.8 Because efficacy and safety outcomes for ravulizumab are similar to those for eculizumab, the cost-effectiveness of ravulizumab will depend on patient preferences concerning the dosing schedule, symptoms related to breakthrough hemolysis, and the final acquisition cost. Key next steps include seeking FDA approval and evaluating the efficacy of ravulizumab in atypical hemolytic uremic syndrome, another key patient population for complement inhibitor therapy. Although none of the patients in the phase 3 studies developed meningococcal infection, it is important to note that 2 patients in the earlier phase 1b/2 studies did develop meningococcus, so clinicians will need to remain vigilant regarding vaccination, antimicrobial prophylaxis, and ongoing surveillance in any patient receiving complement inhibitor therapy.
Overall, ravulizumab is noninferior to eculizumab for treating PNH in patients naive to complement inhibitor therapy and those who previously received eculizumab. With a favorable dosing schedule and fewer episodes of breakthrough hemolysis, ravulizumab is an attractive option for patients with PNH who require complement inhibitor therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.