In this issue of Blood, Winkler et al1 report that extending eltrombopag therapy to at least 24 weeks can improve hematologic responses in patients with refractory severe aplastic anemia (rSAA), a lymphocyte-mediated bone marrow failure syndrome. Genetic analysis of patients from this and the initial rSAA cohorts2,3 warn of cytogenetic evolution in 18% of eltrombopag-treated rSAA patients.
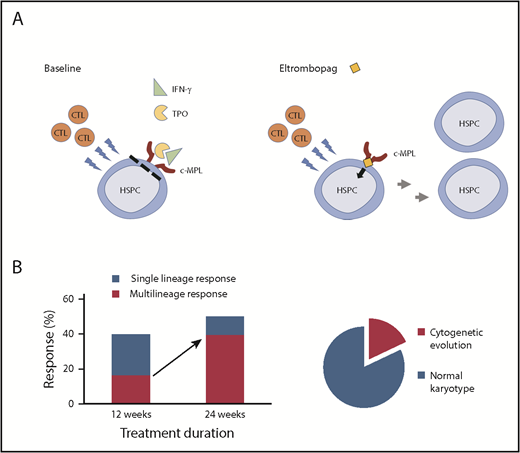
(A) In acquired aplastic anemia, cytotoxic T lymphocytes (CTLs) attack HSPCs, leading to bone marrow aplasia. Elevated levels of inflammatory cytokines cause further bone marrow suppression, in part by interferon-γ (IFN-γ)–mediated inhibition of the endogenous thrombopoietin (TPO) signaling through the c-MPL receptor. A small molecule thrombopoietin mimetic eltrombopag is not affected by IFN-γ and can stimulate c-MPL signaling leading to HSPC recovery. (B) Extending the course of eltrombopag therapy from 12 to 24 weeks in patients with rSAA increased hematologic responses from 40% to 50% and nearly doubled the rate of multilineage hematologic responses. Eighteen percent of rSAA patients treated with eltrombopag developed chromosomal abnormalities within the 24-week study period.
(A) In acquired aplastic anemia, cytotoxic T lymphocytes (CTLs) attack HSPCs, leading to bone marrow aplasia. Elevated levels of inflammatory cytokines cause further bone marrow suppression, in part by interferon-γ (IFN-γ)–mediated inhibition of the endogenous thrombopoietin (TPO) signaling through the c-MPL receptor. A small molecule thrombopoietin mimetic eltrombopag is not affected by IFN-γ and can stimulate c-MPL signaling leading to HSPC recovery. (B) Extending the course of eltrombopag therapy from 12 to 24 weeks in patients with rSAA increased hematologic responses from 40% to 50% and nearly doubled the rate of multilineage hematologic responses. Eighteen percent of rSAA patients treated with eltrombopag developed chromosomal abnormalities within the 24-week study period.
Until the recent approval of eltrombopag, “grave” was an apt description of the prognosis of SAA patients refractory to standard immunosuppressive therapy (IST) with antithymocyte globulin and cyclosporine A. Salvage therapies for patients ineligible for bone marrow transplant were limited to modest efficacy options, such as androgens and alternative immunosuppressants.4 In 2012, a breakthrough phase 1/2 study demonstrated that a 12- to 16-week treatment with a small molecule thrombopoietin mimetic eltrombopag could produce hematologic responses in ∼40% of rSAA patients, including several bi- and trilineage hematologic responses.2,3 The mechanism of multilineage responses to eltrombopag is believed to be mediated by thrombopoietin receptor c-MPL signaling in the remaining hematopoietic stem and progenitor cells (HSPCs) (see figure). The surprising efficacy of eltrombopag despite the already elevated endogenous thrombopoietin levels in SAA patients was explained by the recent discovery of steric inhibition of endogenous thrombopoietin signaling by interferon-γ, which is bypassed by eltrombopag.5
Extended follow-up of patients in the initial National Institutes of Health cohort,2 as well as the emerging real-world experience with eltrombopag in Europe,6 suggested that longer treatment with eltrombopag may improve response rates in rSAA and could rescue patients who would have been deemed refractory after 3 or 4 months of therapy. To evaluate the effectiveness of extended therapy, Winkler et al treated 40 rSAA patients for 24 weeks with a 150-mg daily dose of eltrombopag. The authors found that extending therapy from 12 to 24 weeks improved hematologic responses from 40% to 50% and nearly doubled the rate of multilineage responses, including higher neutrophil counts (see figure). Many responding patients continued eltrombopag beyond 24 weeks, and, remarkably, 9 of the 20 responders (>20% of the study cohort) eventually met the complete response criteria.
Despite these successes, concerns remain about the long-term health of HSPCs recovered with eltrombopag in patients with rSAA. The initial National Institutes of Health cohort had an alarmingly high rate of early cytogenetic evolution,2,3 also confirmed by this study. Nearly 1 in 5 patients (18%) developed chromosomal abnormalities or overt myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) transformation within the 24-week treatment period. Most of the cytogenetic changes appeared within the first 12 weeks of eltrombopag therapy, and nearly one-half involved a complete or a partial loss of chromosome 7. The 18% rate of cytogenetic evolution over a 6-month study period exceeds the expected rate of chromosomal abnormalities in SAA. Historical studies found cytogenetic abnormalities in approximately 10% of aplastic anemia patients, ranging from 3% to 26%.7 The high rate of cytogenetic abnormalities, particularly those involving chromosome 7, in close temporal association with eltrombopag treatment, suggests a causative link between thrombopoietin receptor signaling and cytogenetic evolution. Possible underlying mechanisms include an eltrombopag-mediated selection of preexisting cytogenetically aberrant cells or an increase in genetic instability by stimulating HSPCs beyond the limits of replicative senescence. Alternatively, some rSAA patients may have an occult inherited bone marrow failure syndrome, rendering them both refractory to immunosuppression and potentially more likely to develop genetic instability.
In contrast to cytogenetic abnormalities, the prevalence of somatic mutations was unchanged during the 6-month eltrombopag therapy, and no significant clonal expansion of pathogenic variants was seen within the cohort overall. Interestingly, nearly one-half of the patients did have the emergence of new or the disappearance of previously detected variants, indicative of a dynamic hematopoietic environment. Importantly, aside from abnormalities of chromosome 7, the clinical significance of other changes is unclear. Most patients had no immediate clinical sequela and no morphologic findings of myelodysplasia at the 24-week primary end point.
The crucial unanswered question, beyond the time frame of the present study, concerns the impact of eltrombopag on the long-term risk of secondary MDS and AML in rSAA. Historical studies have shown that 15% to 20% of AA patients develop MDS/AML by 10 years of follow-up.8,9 These data may not accurately reflect the incidence of secondary MDS/AML in a more contemporary SAA cohort, and, particularly, for the higher risk rSAA patients. An ideal study to assess the effect of eltrombopag on late clonal complications in rSAA would include a decade of prospective follow-up of patients randomized to eltrombopag vs best available therapy. At present, the poor prognosis of rSAA and a limited number of effective treatments may preclude such a study for practical and ethical reasons. Long-term follow-up of this and other rSAA cohorts, as well as careful postmarket drug safety surveillance, will thus be of great importance. For newly diagnosed SAA, a randomized controlled trial of eltrombopag added to IST in treatment-naïve SAA patients is currently ongoing and will be informative, although its results may not be directly generalizable to rSAA patients.
Where does this place eltrombopag in the rSAA armamentarium in 2019? Winkler et al established the efficacy of the extended eltrombopag therapy in restoring multilineage hematopoiesis in rSAA. This success comes at a price of new cytogenetic abnormalities in 18% of eltrombopag-treated rSAA patients, nearly one-half of which involve partial or complete loss of chromosome 7. Patients should be screened for cytogenetic abnormalities both before eltrombopag initiation and at regular intervals during therapy; in the absence of safety data, eltrombopag should be avoided in patients with cytogenetic abnormalities. With improved outcomes of allogeneic bone marrow transplantation, transplant-eligible patients should be carefully considered for allogeneic transplantation as an alternative to nontransplant salvage therapies. Although Winkler et al evaluated eltrombopag monotherapy for rSAA, adding eltrombopag to second-line immunosuppression such as rabbit antithymocyte globulin/cyclosporine A may prove to be more efficacious. As more SAA patients receive eltrombopag and IST upfront,10 future questions will include the efficacy and safety of eltrombopag retreatment of relapsed SAA, management of patients who were refractory to upfront eltrombopag and IST, and the long-term effect of cumulative eltrombopag exposure including the role and safety of higher eltrombopag doses and longer treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal