In this issue of Blood, Yin et al demonstrated that engineered BCL6 interacting corepressor protein (Bcor) mutation in hematopoietic stem/progenitor cells of sensitized mice induced acute lymphoid leukemia (ALL) of B-1 progenitor phenotype,1 which has been recently identified by them.2 The intriguing aspect of this report is the complete penetrance of B-1 progenitor leukemia by Bcor mutation, which selectively developed among other various progenitors in the bone marrow (BM).
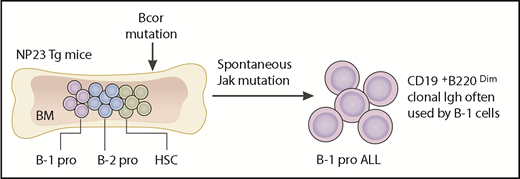
Yin et al engineered a Bcor mutation in BM cells or fetal liver cells from NP23 Tg mice and injected them into lethally irradiated mice. All the recipient mice developed leukemia with B-1 progenitor phenotype and the same Igh usage with B-1 cells. B-1 pro, B-1 progenitors; B-2 pro, B-2 progenitors.
Yin et al engineered a Bcor mutation in BM cells or fetal liver cells from NP23 Tg mice and injected them into lethally irradiated mice. All the recipient mice developed leukemia with B-1 progenitor phenotype and the same Igh usage with B-1 cells. B-1 pro, B-1 progenitors; B-2 pro, B-2 progenitors.
B-1 progenitor ALL or B-1 cells may be unfamiliar terms for many researchers and hematologists. B-1 cells are a unique B-cell subset that is well characterized in mice but not in humans. Unlike conventional adaptive immune B cells (B-2 cells), innate immune-like B-1 cells reside mainly in the pleural and peritoneal cavities. B-1 cells spontaneously secrete natural immunoglobulin M antibodies with stereotypic usage of immunoglobulin heavy chains (Igh), independent of T-cell help. These cells play important roles in the first line of defense against infections.
B-1 cells are unique in their developmental origins. They are mostly derived from progenitors in the fetal liver and neonatal BM and are developmentally different from conventional B-2 cells that are differentiated from hematopoietic stem cells (HSCs) through B-2 progenitors (such as prepro-B, pro-B, and pre-B cells) in the BM.3,4 B-1–specific progenitors, sIg−AA4.1+CD19+B220lo/− (CD19 single positive) population has been identified in the fetal liver and neonatal BM, and the number of the B-1–specific progenitors in the postnatal BM declines with mouse age.5,6 B-1–specific progenitors are segregated from conventional B-2 progenitors, sIg−AA4.1+CD19-B220+ (B220 single positive) population. Both the progenitors mature into sIg−CD19+B220+ (CD19B220 double positive) pre-B cells, and thus, after the pre-B-cell stage, one cannot distinguish B-1 progenitors from B-2 progenitors, and B-1 progenitors are a very small population in the adult BM. Importantly, the human counterpart of mouse B-1 cells has been controversial.
Almost all acute leukemias of B-progenitor phenotype in mouse models are thought to be the B-2 progenitor-derived, expressing surface markers of pro-B/pre-B cells (B220 single-positive or CD19B220 double-positive cells). However, the B-progenitor leukemia induced by Bcor mutation in NUP98-PHF23 (NP23) transgenic (Tg) mice, reported by Yin et al, displayed a CD19 single-positive B-1–specific progenitor phenotype, accompanied by a spontaneous Jak mutation (see figure).
NP23 is a chromatin-modifying oncoprotein, associated with human acute myeloid leukemia (AML). Previously, it has been reported that all NP23 Tg mice develop leukemia, including AML, T-cell ALL, and B-cell ALL, by 14 months of age.2,7 B-1 progenitor leukemia, with spontaneous mutations of Bcor and Jak, was found in ∼10% of these leukemic mice. Intriguingly, not only the surface markers but also the clonal Igh usage of B-1 progenitor leukemic cells are similar to fetal liver–derived B-1 cells.2 Based on these results, Yin et al engineered a Bcor mutation by Cas9 in lineage-negative hematopoietic progenitors from the BM and the fetal liver of NP23 Tg mice and transplanted them into irradiated mice. Surprisingly, B-1 progenitor leukemia developed in all recipient mice. B-1 progenitors are a very small population among other progenitors in the BM, including HSCs, myeloid progenitors, common lymphoid progenitors, and B-2 pro/pre-B progenitors (see figure). Why do only B-1 progenitors develop leukemia when Bcor mutation was engineered in total NP23 Tg BM cells? It is generally considered that leukemogenic events are not sufficient to induce leukemia in all blood cells; rather, they need to occur in the selective hematopoietic lineage and at a specific progenitor stage in order to develop leukemia.8 Therefore, the results by Yin et al suggest that there must be an underlying mechanism through which Bcor mutation selectively induced B-1 progenitor leukemia with spontaneous Jak mutation.
An animal model of acute leukemia with B-1 progenitor phenotype has not been reported before. There is a report showing that B-1 progenitors developed more aggressive ALL with shorter latency compared with B-2 progenitors when the BCR-ABL gene was overexpressed in B-1 and B-2 progenitors and the cells were transplanted into immunodeficient mice.9 The BCR-ABL leukemic cells in recipient mice displayed CD19B220 double-positive pre-B-cell phenotype regardless of the B-1 or B-2 progenitor origin. Because both B-1 and B-2 progenitors become CD19B220 double-positive cells during maturation, this result suggests that CD19B220 double-positive pre-B leukemia may include both B-1 and B-2 progenitor cell origins. In this sense, the report by Yin et al supports the possibility that B-lymphoid leukemia of B-1 progenitor origin may be underappreciated.
Another important aspect of this and their previous report is that we may be able to connect mouse B-1 progenitor leukemia with the human counterpart. The Bcor mutation in human B-ALL is very rare; however, a correlation between mouse B-1 progenitor ALL in NP23 Tg and human B-progenitor ALL with Jak mutation and cytokine receptor like factor 2 (CRLF2) overexpression has been indicated by gene expression signature and the preference for Igh usage.2 CRLF2 encodes a receptor for thymic stromal lymphopoietin, an important cytokine receptor for B-1 cell development. Therefore, the B-1 progenitor ALL mouse model could be a great tool to identify the human counterpart of B-1 progenitors and related leukemias. Because the cell surface markers are quite different between mice and human, identifying the human counterpart of B-1 cells has been challenging and still controversial. Elucidating the underlying mechanism via which B-1 progenitors selectively develop leukemia would be a key to resolve this long-standing question in the field.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal