Key Points
The MTSS includes clinical-molecular and transplant-specific factors predicting posttransplant outcome.
The MTSS is applicable to primary and post-ET/PV myelofibrosis reflecting posttransplant outcome better than disease-specific systems.
Abstract
Allogeneic hematopoietic stem cell transplantation is curative in myelofibrosis, and current prognostic scoring systems aim to select patients for transplantation. Here, we aimed to develop a prognostic score to determine prognosis after transplantation itself, using clinical, molecular, and transplant-specific information from a total of 361 patients with myelofibrosis. Of these, 205 patients were used as a training cohort to create a clinical-molecular myelofibrosis transplant scoring system (MTSS), which was then externally validated in a cohort of 156 patients. Multivariable analysis on survival identified age at least 57 years, Karnofsky performance status lower than 90%, platelet count lower than 150 × 109/L, leukocyte count higher than 25 × 109/L before transplantation, HLA-mismatched unrelated donor, ASXL1 mutation, and non-CALR/MPL driver mutation genotype being independent predictors of outcome. The uncorrected concordance index for the final survival model was 0.723, and bias-corrected indices were similar. Risk factors were incorporated into a 4-level MTSS: low (score, 0-2), intermediate (score, 3-4), high (score, 5), and very high (score, >5). The 5-year survival according to risk groups in the validation cohort was 83% (95% confidence interval [CI], 71%-95%), 64% (95% CI, 53%-75%), 37% (95% CI, 17%-57%), and 22% (95% CI, 4%-39%), respectively (P < .001). Increasing score was predictive of nonrelapse mortality (P < .001) and remained applicable to primary (0.718) and post-essential thrombocythemia (ET)/polycythemia vera (PV) myelofibrosis (0.701) improving prognostic ability in comparison with all currently available disease-specific systems. In conclusion, this MTSS predicts outcome of patients with primary and post-ET/PV myelofibrosis undergoing allogeneic stem cell transplantation.
Introduction
Despite the approval of Janus kinase inhibitor treatment in myelofibrosis, allogeneic stem cell transplantation remains the only curative treatment option for myelofibrosis, whereas the transplant procedure itself still has high therapy-related morbidity and mortality, despite recent improvements; and because of the variable outcome of patients with myelofibrosis, treatment decision with respect to allogeneic stem cell transplantation should be based on a careful risk-benefit analysis.1-3
Current prognostic scoring systems aim to determine who among patients with myelofibrosis should be referred to transplantation, and were thus developed in diagnosed patients with either primary myelofibrosis (PMF) or post-essential thrombocythemia (ET) or polycythemia vera (PV) myelofibrosis. In PMF, the International Prognostic Scoring System (IPSS) is valid only for newly diagnosed patients, whereas the dynamic IPSS (DIPSS) showed applicability at all times of the disease course.4,5 The IPSS and DIPSS both include 5 independent variables predicting survival (age >65 years, hemoglobin <10 g/dL, leukocytes >25 × 109/L, circulating blasts ≥1%, and constitutional symptoms), whereas the DIPSS-plus score also considered 3 additional prognostic factors (transfusion-dependence, platelet count <100 × 109/L, and unfavorable karyotype).6 Furthermore, the prognostic relevance of mutation profile resulted in a mutation-enhanced system (MIPSS70) in transplant-age patients with PMF (70 years or younger) incorporating CALR type 1 mutation; presence of ASXL1, EZH2, SRSF2, or IDH1/2 mutations; as well as the number of high-risk mutations, a refinement including a 3-tiered cytogenetic risk classification (MIPSS70-plus version 2.0) and a system only focusing on genetic factors (GIPSS).7-12 For patients with post-ET/PV myelofibrosis, the Myelofibrosis Secondary to Polycythemia Vera and Essential Thrombocythemia-Prognostic Model (MYSEC-PM) was developed including hemoglobin level lower than 11 g/dL, circulating blasts higher than 2%, a CALR-unmutated genotype, platelet count lower than 150 × 109/L, age, and the presence of constitutional symptoms.13
However, uncertainty remains regarding the usefulness of current systems to predict outcome after transplantation itself.14-17 In a matched analysis of patients with PMF treated with transplantation or conventional therapy in the preruxolitinib era, patients with intermediate 2 risk and high risk according to DIPSS showed improved survival after transplantation, whereas low-risk patients benefitted more likely from a nontransplant approach and intermediate-1-risk patients were in favor neither for transplant nor for a nontransplant approach.18 To predict posttransplant outcome, transplant-specific factors such as intensity of the conditioning regimen, different recipient age categories, cytomegalovirus serostatus, performance status, or HLA matching of the donor may be considered.19-22
Here, we aimed to develop a comprehensive clinical-molecular model for myelofibrosis to predict outcome after stem cell transplantation, which may allow proper counseling regarding patients’ posttransplant prognosis.
Methods
Patients
We included 361 patients with myelofibrosis undergoing first allogeneic stem cell transplantation, of which 260 were diagnosed with PMF at time of transplantation, whereas the remaining 101 patients had either post-ET (n = 55) or post-PV myelofibrosis (n = 46); 164 patients were recruited from the University Medical Center Hamburg-Eppendorf (Hamburg, Germany), 128 from the West German Cancer Center (Essen, Germany), 41 from the Hôpital Saint-Louis (Paris, France), and 28 from the Hannover Medical School (Hannover, Germany). Patients with myelofibrosis progressed to acute leukemia were excluded. Acute leukemia was defined as at least 20% blasts in peripheral blood or bone marrow. Very few patients had between 10% and 20% peripheral blasts, whereas all of them had less than 5% blasts in bone marrow histology, and thus, all were classified as myelofibrosis chronic phase.
The training cohort consisted of 205 patients from Hamburg and Paris, whereas the remaining 156 patients from Essen and Hannover were included in the validation cohort. Clinical and transplant-specific variables and samples for sequencing and cytogenetic analyses were collected at time of transplantation at each center. This study was conducted in accordance with the Declaration of Helsinki, and scientific analysis of the samples was approved by institutional review boards.
Mutational and cytogenetic analyses
Bone marrow or peripheral blood samples were obtained before transplantation, and mutations were detected using next-generation sequencing, as previously described.16 The following myelofibrosis-associated genes were sequenced: JAK2, CALR, MPL, ASXL1, IDH1/2, CBL, DNMT3A, TET2, SF3B1, SRSF2, U2AF1, EZH2, TP53, NRAS, KRAS, RUNX1, and FLT3. Cytogenetic analysis in PMF or post-ET/PV myelofibrosis is not performed routinely in Europe, and thus complete cytogenetic data were available only in 202 patients (60%). Cytogenetic reporting was performed according to the International System for Human Cytogenetic Nomenclature criteria, using standardized techniques.23
Statistical analysis
A 2-step method was used to develop a comprehensive clinical-molecular myelofibrosis transplant scoring system (MTSS). In a first step, variables associated with overall survival (OS) at P ≤ .10 in univariable analysis were selected and then, subsequently, used to construct a multivariable Cox proportional hazards model identifying independent prognostic factors that will be included in a prognostic system.24 All variables included in previous prognostic systems were assessed, as well as additional clinical and transplant-specific variables such as Karnofsky performance status, spleen status before transplantation, time between diagnosis and transplantation, stem cell graft source, cytomegalovirus serostatus of patient and donor, and donor source (HLA-matched related, matched unrelated, mismatched unrelated, or mismatched related including haploidentical).
The role of underlying disease (PMF or post-ET/PV myelofibrosis) for the model was determined a priori as follows: first, no significant difference in survival was identified in univariable analysis (P = .647); second, we then considered PMF and post-ET/PV myelofibrosis as a stratifying variable investigating the performance of the model according to both diseases, which may then enable appropriate comparisons to all existing models. Moreover, pretreatment with ruxolitinib was excluded from model analysis because of a too short follow-up for patients receiving ruxolitinib (median, 2.5 years).
OS was defined as time from date of transplantation to death from any cause. The distribution of OS was estimated by the Kaplan-Meier method and compared by log-rank test.25 Cutoffs for continuous variables were established by using results of the likelihood ratio test. All 2-way interactions were evaluated, and the final model for OS was selected on the basis of clinical judgment and by comparison of Akaike information criterion. The final OS model was internally validated by use of bootstrap resampling and cross-validation methods, leaving 5 or 10 samples out at each iteration, and served as the basis for creation of the model.26 As is standard, points assigned to each variable included in the risk model were assigned proportional to the weights of resulting hazard ratios (HRs).
The accuracy of prediction of OS was evaluated by estimating the model’s discrimination measured by the concordance index (C-index).26 The C-index is the probability that for 2 randomly selected patients, the patient who experienced the event first had a higher probability of having the event, according to the model. A C-index of 0.5 represents agreement by chance alone, and a C-index of 1 means perfect discrimination.
The model was then applied to the secondary outcome of nonrelapse mortality (NRM), using cause-specific HRs in a competing events framework. These results were confirmed using the Fine and Gray method to account for competing risks.27 All values with P < .05 were considered statistically significant, and all analyses were performed using R software version 3.4.3 (https://www.r-project.org/).
Results
Patients
The study included 205 patients with PMF and post-ET/PV myelofibrosis undergoing first allogeneic stem cell transplantation, which were used as a training cohort to develop the MTSS, whereas 156 were included in the validation cohort. Patient data for the total cohort, as well as for the training and validation cohort, are listed in Table 1. Patients diagnosed with post-ET/PV myelofibrosis at time of transplantation, at younger age, with absence of constitutional symptoms, and subsequently with lower risk according to DIPSS, MIPSS70, and MYSEC-PM were enriched in the validation cohort. Transplantations in the training cohort were mainly applied using a reduced intensity regimen, whereas most patients in the validation cohort received a myeloablative conditioning regimen. The median time between diagnosis and transplantation was 23 months, and median follow-up time was 5.2 years. The 5-year OS and NRM rates were 62% (95% CI, 55%-69%) and 28% (95% CI, 23%-33%) in the training cohort and 58% (95% CI, 50%-66%) and 31% (95% CI, 27%-35%) in the validation cohort, respectively (P = .36 and .45).
Characteristics of all patients with myelofibrosis and of the training and validation cohorts undergoing allogeneic stem cell transplantation.
| Characteristic . | Total cohort (n = 361) . | Training cohort (n = 205) . | Validation cohort (n = 156) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| Median (range) | 56 (18-75) | 57 (29-75) | 55 (18-70) | <.001 |
| Male sex | 211 (58) | 122 (59) | 89 (57) | .667 |
| Diagnosis before transplant | .237 | |||
| PMF | 260 (72) | 153 (75) | 107 (69) | |
| Post-ET/PV | 101 (28) | 52 (25) | 49 (31) | |
| Blood levels, median (range) | ||||
| Hemoglobin, g/dL | 9.5 (5.6-17.9) | 9.5 (5.6-17.9) | 9.5 (5.6-17.7) | .549 |
| Leukocytes, ×109/L | 8.1 (0.4-168.8) | 9.1 (0.8-168.8) | 7.5 (0.4-103.0) | .098 |
| Platelets, ×109/L | 150 (4-2513) | 171 (5-2437) | 124 (4-2513) | .154 |
| Peripheral blasts, % | 1 (0-19) | 1 (0-19) | 1 (0-19) | .271 |
| BM fibrosis grade >1 | 288 (80) | 172 (84) | 116 (74) | .016 |
| KPS, % | .284 | |||
| 90-100 | 208 (58) | 113 (55) | 95 (61) | |
| <90 | 153 (42) | 92 (45) | 61 (39) | |
| Constitutional symptoms | 208 (58) | 148 (73) | 60 (39) | <.001 |
| Transfusion dependence | 150 (42) | 97 (47) | 53 (34) | .002 |
| Cytogenetics | 202 (56) | 150 (73) | 52 (33) | <.001 |
| Driver mutation | .017 | |||
| CALR | 73 (20) | 44 (22) | 29 (19) | |
| MPL | 18 (5) | 10 (5) | 8 (5) | |
| JAK2 | 206 (57) | 126 (62) | 80 (51) | |
| Triple negative | 64 (18) | 25 (12) | 39 (25) | |
| Number of mutations | <.001 | |||
| 0-3 | 303 (84) | 157 (77) | 146 (94) | |
| >3 | 58 (16) | 48 (23) | 10 (6) | |
| DIPSS* | .008 | |||
| Low | 23 (9) | 8 (5) | 15 (14) | |
| Intermediate 1 | 80 (31) | 42 (28) | 38 (36) | |
| Intermediate 2 | 120 (46) | 75 (49) | 45 (42) | |
| High | 37 (14) | 28 (18) | 9 (8) | |
| MIPSS* | <.001 | |||
| Low | 4 (1) | 0 (0) | 4 (4) | |
| Intermediate | 88 (34) | 41 (27) | 47 (44) | |
| High | 168 (65) | 112 (73) | 56 (52) | |
| MYSEC-PM† | .063 | |||
| Low | 24 (24) | 7 (14) | 17 (35) | |
| Intermediate 1 | 39 (38) | 23 (44) | 16 (33) | |
| Intermediate 2 | 25 (25) | 13 (25) | 12 (25) | |
| High | 13 (13) | 9 (17) | 4 (8) | |
| Time to transplant, months | ||||
| Median (range) | 23.3 (0.5-526.5) | 28.4 (0.5-526.5) | 20.0 (1.5-305.3) | .234 |
| Conditioning intensity | <.001 | |||
| Reduced | 230 (64) | 196 (96) | 34 (22) | |
| Myeloablative | 131 (36) | 9 (4) | 122 (78) | |
| CMV status patient/donor | .157 | |||
| −/− | 104 (29) | 63 (31) | 41 (26) | |
| −/+ | 41 (11) | 20 (10) | 21 (13) | |
| +/− | 51 (14) | 23 (11) | 28 (18) | |
| +/+ | 165 (46) | 99 (48) | 66 (42) | |
| HLA-match | .017 | |||
| Matched related | 96 (26) | 51 (25) | 45 (29) | |
| Matched unrelated | 165 (46) | 86 (42) | 79 (51) | |
| Mismatched related | 4 (1) | 1 (1) | 3 (2) | |
| Mismatched unrelated | 96 (26) | 67 (33) | 29 (19) | |
| Graft source | .978 | |||
| PB | 347 (96) | 197 (96) | 150 (96) | |
| BM | 14 (4) | 8 (4) | 6 (4) | |
| Splenectomy before transplant | 48 (13) | 26 (13) | 22 (14) | .755 |
| Ruxolitinib before transplant | 79 (22) | 48 (24) | 31 (20) | .520 |
| Characteristic . | Total cohort (n = 361) . | Training cohort (n = 205) . | Validation cohort (n = 156) . | P . |
|---|---|---|---|---|
| Age, y | ||||
| Median (range) | 56 (18-75) | 57 (29-75) | 55 (18-70) | <.001 |
| Male sex | 211 (58) | 122 (59) | 89 (57) | .667 |
| Diagnosis before transplant | .237 | |||
| PMF | 260 (72) | 153 (75) | 107 (69) | |
| Post-ET/PV | 101 (28) | 52 (25) | 49 (31) | |
| Blood levels, median (range) | ||||
| Hemoglobin, g/dL | 9.5 (5.6-17.9) | 9.5 (5.6-17.9) | 9.5 (5.6-17.7) | .549 |
| Leukocytes, ×109/L | 8.1 (0.4-168.8) | 9.1 (0.8-168.8) | 7.5 (0.4-103.0) | .098 |
| Platelets, ×109/L | 150 (4-2513) | 171 (5-2437) | 124 (4-2513) | .154 |
| Peripheral blasts, % | 1 (0-19) | 1 (0-19) | 1 (0-19) | .271 |
| BM fibrosis grade >1 | 288 (80) | 172 (84) | 116 (74) | .016 |
| KPS, % | .284 | |||
| 90-100 | 208 (58) | 113 (55) | 95 (61) | |
| <90 | 153 (42) | 92 (45) | 61 (39) | |
| Constitutional symptoms | 208 (58) | 148 (73) | 60 (39) | <.001 |
| Transfusion dependence | 150 (42) | 97 (47) | 53 (34) | .002 |
| Cytogenetics | 202 (56) | 150 (73) | 52 (33) | <.001 |
| Driver mutation | .017 | |||
| CALR | 73 (20) | 44 (22) | 29 (19) | |
| MPL | 18 (5) | 10 (5) | 8 (5) | |
| JAK2 | 206 (57) | 126 (62) | 80 (51) | |
| Triple negative | 64 (18) | 25 (12) | 39 (25) | |
| Number of mutations | <.001 | |||
| 0-3 | 303 (84) | 157 (77) | 146 (94) | |
| >3 | 58 (16) | 48 (23) | 10 (6) | |
| DIPSS* | .008 | |||
| Low | 23 (9) | 8 (5) | 15 (14) | |
| Intermediate 1 | 80 (31) | 42 (28) | 38 (36) | |
| Intermediate 2 | 120 (46) | 75 (49) | 45 (42) | |
| High | 37 (14) | 28 (18) | 9 (8) | |
| MIPSS* | <.001 | |||
| Low | 4 (1) | 0 (0) | 4 (4) | |
| Intermediate | 88 (34) | 41 (27) | 47 (44) | |
| High | 168 (65) | 112 (73) | 56 (52) | |
| MYSEC-PM† | .063 | |||
| Low | 24 (24) | 7 (14) | 17 (35) | |
| Intermediate 1 | 39 (38) | 23 (44) | 16 (33) | |
| Intermediate 2 | 25 (25) | 13 (25) | 12 (25) | |
| High | 13 (13) | 9 (17) | 4 (8) | |
| Time to transplant, months | ||||
| Median (range) | 23.3 (0.5-526.5) | 28.4 (0.5-526.5) | 20.0 (1.5-305.3) | .234 |
| Conditioning intensity | <.001 | |||
| Reduced | 230 (64) | 196 (96) | 34 (22) | |
| Myeloablative | 131 (36) | 9 (4) | 122 (78) | |
| CMV status patient/donor | .157 | |||
| −/− | 104 (29) | 63 (31) | 41 (26) | |
| −/+ | 41 (11) | 20 (10) | 21 (13) | |
| +/− | 51 (14) | 23 (11) | 28 (18) | |
| +/+ | 165 (46) | 99 (48) | 66 (42) | |
| HLA-match | .017 | |||
| Matched related | 96 (26) | 51 (25) | 45 (29) | |
| Matched unrelated | 165 (46) | 86 (42) | 79 (51) | |
| Mismatched related | 4 (1) | 1 (1) | 3 (2) | |
| Mismatched unrelated | 96 (26) | 67 (33) | 29 (19) | |
| Graft source | .978 | |||
| PB | 347 (96) | 197 (96) | 150 (96) | |
| BM | 14 (4) | 8 (4) | 6 (4) | |
| Splenectomy before transplant | 48 (13) | 26 (13) | 22 (14) | .755 |
| Ruxolitinib before transplant | 79 (22) | 48 (24) | 31 (20) | .520 |
Data are given as no. (%) except when specified otherwise.
BM, bone marrow; CMV, cytomegalovirus; KPS, Karnofsky performance status; PB, peripheral blood.
n = 260 (only PMF).
n = 101 (only post-ET/PV myelofibrosis).
A driver mutation was found in 88% and 75% of patients in the training and validation cohort, respectively: JAK2 in 62% and 51%, CALR type 1 in 14% and 13%, CALR type 2 in 6% and 3%, other CALR type in 2% and 3%, MPL in 5% and 5%, and triple negative in 12% and 25%. Of all mutations, 23% of the training cohort and 6% of the validation cohort had more than 3 mutations. The most frequent mutations were ASXL1 (38% and 24%), TET2 (18% and 20%), SRSF2 (9%, respectively), and DNMT3A (5% and 6%). Clinical, molecular and transplant data were complete, whereas cytogenetic data were available in 73% of the training cohort and 33% of the validation cohort; 17% and 40% had unfavorable karyotype according to DIPSS-plus. According to the 3-tiered cytogenetic risk stratification of the MIPSS70-plus version 2.0, 10% and 7% of the training cohort and 35% and 12% of the validation cohort had unfavorable or very high risk karyotype.
Development of a myelofibrosis transplant scoring system
Clinical, molecular, and transplant-specific variables associated with 5-year OS at P ≤ .10 in the training cohort of 205 patients with myelofibrosis were used to construct a multivariate Cox proportional hazards model in which the effect of each covariate was adjusted for that of all others. The following variables met the predetermined significance level: older age, leukocytosis, thrombocytopenia, HLA-mismatched unrelated donor, cytomegalovirus serostatus positive patient and negative donor, non-CALR/MPL driver mutation genotype, more than 3 mutations overall, ASXL1, DNMT3A, Karnofsky performance status lower than 90%, and the presence of constitutional symptoms. Table 2 summarizes the variables relevant to OS identified in the univariable and multivariable analysis of the 205 patients in the training cohort.
OS model
| Variable . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Leukocyte count >25 × 109/L | 1.62 (1.10-2.41) | .015 | 1.57 (1.16-2.41) | .007 |
| Platelet count <150 × 109/L | 1.89 (1.17-3.05) | .009 | 1.67 (1.16-2.40) | .006 |
| Peripheral blasts >1% | 1.03 (0.63-1.66) | .918 | ||
| Peripheral blasts (continuous) | 1.02 (0.93-1.11) | .696 | ||
| Hemoglobin <10 g/dL | 1.13 (0.70-1.84) | .617 | ||
| KPS <90% | 1.47 (1.05-2.06) | .026 | 1.50 (1.06-2.13) | .021 |
| Constitutional symptoms | 1.35 (0.95-1.92) | .092 | ||
| Transfusion dependence | 1.15 (0.81-1.64) | .423 | ||
| BM fibrosis grade >1 | 1.00 (0.66-1.53) | .999 | ||
| Driver mutation | ||||
| CALR type 1 | Reference | |||
| CALR type 2 | 1.05 (0.38-2.92) | .929 | ||
| MPL | 0.52 (0.07-4.17) | .540 | ||
| JAK2 | 2.67 (1.26-5.60) | .010 | ||
| Triple negative | 3.02 (1.19-7.67) | .020 | ||
| CALR or MPL | ||||
| Present | Reference | |||
| Absent | 2.97 (1.48-6.01) | .002 | 2.40 (1.30-4.71) | .012 |
| Age ≥57 y | 2.69 (1.59-4.56) | <.001 | 1.65 (1.15-2.36) | .006 |
| HLA-mismatched unrelated | 1.99 (1.40-2.82) | <.001 | 2.08 (1.45-2.97) | <.001 |
| HLA-match | ||||
| Matched related | Reference | |||
| Matched unrelated | 1.24 (0.75-1.93) | .303 | ||
| Mismatched related | 1.08 (0.15-7.91) | .943 | ||
| Mismatched unrelated | 2.41 (1.51-3.84) | <.001 | ||
| ASXL1 | 1.50 (1.13-2.25) | .018 | 1.42 (1.01-2.01) | .041 |
| U2AF1* | 1.48 (0.70-3.07) | .309 | ||
| DNMT3A† | 1.58 (0.90-2.61) | .100 | ||
| TP53‡ | 1.02 (0.14-7.35) | .985 | ||
| Number of mutations >3 | 1.52 (0.92-2.57) | .098 | ||
| High molecular risk¶ | 1.49 (0.89-2.48) | .129 | ||
| Cytogenetic risk (MIPSS70-plus version 2.0) | ||||
| Favorable karyotype | Reference | |||
| Unfavorable karyotype | 1.69 (0.86-3.32) | .126 | ||
| Very high risk karyotype | 0.68 (0.21-2.22) | .526 | ||
| Unfavorable karyotype (DIPSS-plus) | 1.54 (0.79-2.41) | .451 | ||
| CMV serostatus patient/donor | ||||
| −/− | Reference | |||
| −/+ | 0.85 (0.44-1.63) | .616 | ||
| +/− | 1.63 (1.02-2.67) | .045 | ||
| +/+ | 1.09 (0.72-1.66) | .676 | ||
| Time to transplant | 0.99 (0.99-1.00) | .553 | ||
| Ruxolitinib before transplant§ | 0.67 (0.35-1.29) | .228 | ||
| Splenectomy before transplant | 0.93 (0.57-1.53) | .772 | ||
| Variable . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Leukocyte count >25 × 109/L | 1.62 (1.10-2.41) | .015 | 1.57 (1.16-2.41) | .007 |
| Platelet count <150 × 109/L | 1.89 (1.17-3.05) | .009 | 1.67 (1.16-2.40) | .006 |
| Peripheral blasts >1% | 1.03 (0.63-1.66) | .918 | ||
| Peripheral blasts (continuous) | 1.02 (0.93-1.11) | .696 | ||
| Hemoglobin <10 g/dL | 1.13 (0.70-1.84) | .617 | ||
| KPS <90% | 1.47 (1.05-2.06) | .026 | 1.50 (1.06-2.13) | .021 |
| Constitutional symptoms | 1.35 (0.95-1.92) | .092 | ||
| Transfusion dependence | 1.15 (0.81-1.64) | .423 | ||
| BM fibrosis grade >1 | 1.00 (0.66-1.53) | .999 | ||
| Driver mutation | ||||
| CALR type 1 | Reference | |||
| CALR type 2 | 1.05 (0.38-2.92) | .929 | ||
| MPL | 0.52 (0.07-4.17) | .540 | ||
| JAK2 | 2.67 (1.26-5.60) | .010 | ||
| Triple negative | 3.02 (1.19-7.67) | .020 | ||
| CALR or MPL | ||||
| Present | Reference | |||
| Absent | 2.97 (1.48-6.01) | .002 | 2.40 (1.30-4.71) | .012 |
| Age ≥57 y | 2.69 (1.59-4.56) | <.001 | 1.65 (1.15-2.36) | .006 |
| HLA-mismatched unrelated | 1.99 (1.40-2.82) | <.001 | 2.08 (1.45-2.97) | <.001 |
| HLA-match | ||||
| Matched related | Reference | |||
| Matched unrelated | 1.24 (0.75-1.93) | .303 | ||
| Mismatched related | 1.08 (0.15-7.91) | .943 | ||
| Mismatched unrelated | 2.41 (1.51-3.84) | <.001 | ||
| ASXL1 | 1.50 (1.13-2.25) | .018 | 1.42 (1.01-2.01) | .041 |
| U2AF1* | 1.48 (0.70-3.07) | .309 | ||
| DNMT3A† | 1.58 (0.90-2.61) | .100 | ||
| TP53‡ | 1.02 (0.14-7.35) | .985 | ||
| Number of mutations >3 | 1.52 (0.92-2.57) | .098 | ||
| High molecular risk¶ | 1.49 (0.89-2.48) | .129 | ||
| Cytogenetic risk (MIPSS70-plus version 2.0) | ||||
| Favorable karyotype | Reference | |||
| Unfavorable karyotype | 1.69 (0.86-3.32) | .126 | ||
| Very high risk karyotype | 0.68 (0.21-2.22) | .526 | ||
| Unfavorable karyotype (DIPSS-plus) | 1.54 (0.79-2.41) | .451 | ||
| CMV serostatus patient/donor | ||||
| −/− | Reference | |||
| −/+ | 0.85 (0.44-1.63) | .616 | ||
| +/− | 1.63 (1.02-2.67) | .045 | ||
| +/+ | 1.09 (0.72-1.66) | .676 | ||
| Time to transplant | 0.99 (0.99-1.00) | .553 | ||
| Ruxolitinib before transplant§ | 0.67 (0.35-1.29) | .228 | ||
| Splenectomy before transplant | 0.93 (0.57-1.53) | .772 | ||
Akaike information criterion, 688.629; C-index original, 0.723; bootstrap C-index: 0.712.
MIPSS, mutation-enhanced International Prognostic Scoring System.
n = 17 with U2AF1.
n = 11 with DNMT3A.
n = 3 with TP53.
High-molecular-risk category indicates the presence of a mutation in any of the following genes in a patient: ASXL1, EZH2, SRSF2, or IDH1/2; mutation-specific HRs were 1.50 (P = .018) for ASXL1, 0.69 (P = .522) for EZH2, 0.85 (P = .734) for SRSF2, and 0.91 (P = .855) for IDH1/2.
Median follow-up in ruxolitinib and nonruxolitinib cohorts were 2.5 and 5.8 years; HR is shown for 3-year survival.
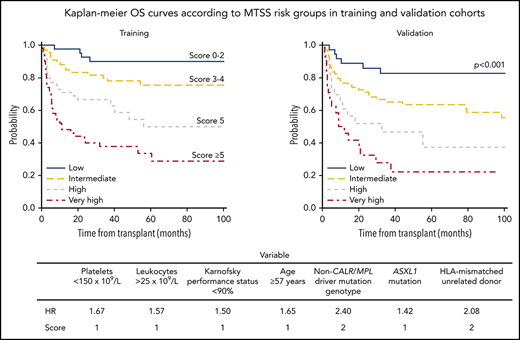
The multivariable model identified 7 independent predictors of survival: age at least 57 years, Karnofsky performance status lower than 90%, a non-CALR/MPL driver mutation genotype, ASXL1 mutation, HLA-mismatch unrelated donor, leukocyte count higher than 25 × 109/L, and platelet count lower than 150 × 109/L before transplantation.
Model discrimination was evaluated with the C-index, which quantifies the level of concordance between the predicted and observed OS. The C-index for the final OS model was 0.723 (95% CI, 0.713-0.733). The bias-corrected C-indices generated by bootstrap validations were 0.712 (95% CI, 0.703-0.721) and 0.719 (95% CI, 0.709-0.729), with five-fold internal cross-validation similar to that of 10-fold internal cross-validation, arguing against an overfit model.
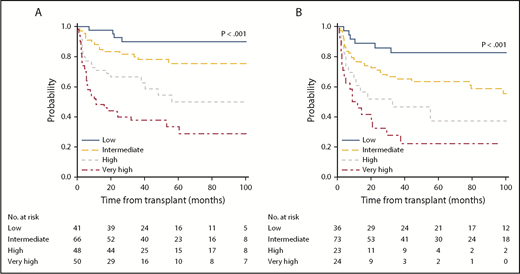
The internally validated model was used to develop a discrete system predicting 5-year OS. Based on an HR of 2 or more, a weighted score of 2 was assigned to transplantation from an HLA-mismatch unrelated donor and a non-CALR/MPL driver mutation genotype, whereas other factors were assigned a score of 1 based on an HR lower than 2. References used to calculate HRs were assigned a score of 0. Subsequently, a score of 1 was assigned to older age (≥57 years), leukocytosis, thrombocytopenia, ASXL1 mutation, and a Karnofsky performance status lower than 90%. The overall score ranged from 0 to 9, with increasing scores indicating greater risk. On the basis of these data, a 4-category system was created: low (score of 0-2), intermediate (score of 3-4), high (score of 5), and very high (score of 6-9). The MTSS was predictive of OS resulting in HRs for death (using the low-risk group as reference) of 2.08 (95% CI, 1.14-3.77) for the intermediate-risk group, 3.72 (95% CI, 2.00-6.94) for the high-risk group, and 6.95 (95% CI, 3.83-12.61) for the very high–risk group (overall P < .001). The corresponding 5-year OS according to each risk group was 90% (low), 77% (intermediate), 50% (high), and 34% (very high; Figure 1A).
5-year overall survival according to MTSS. OS for training (A) and validation cohort (B) according to the MTSS risk stratification. The 5-year survival rates according to each cohort and corresponding risk group were 90% (low), 77% (intermediate), 50% (high), and 34% (very high) for the training cohort (n = 205) and 83% (low), 64% (intermediate), 37% (high), and 22% (very high) for the validation cohort (n = 156; P < .001, respectively).
5-year overall survival according to MTSS. OS for training (A) and validation cohort (B) according to the MTSS risk stratification. The 5-year survival rates according to each cohort and corresponding risk group were 90% (low), 77% (intermediate), 50% (high), and 34% (very high) for the training cohort (n = 205) and 83% (low), 64% (intermediate), 37% (high), and 22% (very high) for the validation cohort (n = 156; P < .001, respectively).
Important variables that were not associated with OS in the multivariable analysis included percentage of peripheral blasts, cytomegalovirus serostatus of patient and donor, DNMT3A and U2AF1 mutations, high-risk mutation category, the number of mutations overall, and the presence of constitutional symptoms.
External validation
To evaluate the OS model generated in the training cohort, the MTSS was applied to a validation cohort of 156 patients. Among these 156 patients, the MTSS was associated with OS (P < .001), with HRs for each risk group (using the low-risk group as reference) being 1.99 (95% CI, 1.01-4.18) for the intermediate-risk, 3.63 (95% CI, 1.54-8.56) for the high-risk, and 6.36 (95% CI, 2.81-14.41) for the very high risk group. The 5-year OS was 83% (low), 64% (intermediate), 37% (high), and 22% (very high; Figure 1B).
Nonrelapse mortality
Because the training set was developed based on OS and no other outcomes, we combined the 205 patients from the training cohort with the 156 patients from the validation cohort for analysis of secondary objectives. In the combined cohort, the MTSS was associated with 5-year NRM (P < .001, respectively) showing hazard ratios (with low-risk as reference) of 2.34 (95% CI, 1.20-4.30) for the intermediate-risk, 4.12 (95% CI, 2.51-6.09) for the high-risk, and 9.28 (95% CI, 5.71-16.99) for the very high risk groups. The 5-year NRM according to each risk group was 10% (low), 22% (intermediate), 36% (high), and 57% (very high).
Comparison with existing systems
To quantify which prognostic system better fit actual outcomes, we calculated a C-index for the MTSS, including 260 patients with PMF for whom complete data were available for the DIPSS and MIPSS70, and 101 patients with post-ET/PV myelofibrosis for MYSEC-PM. Concordance indices describe the probability that predicted and observed survival times are similar among ranked pairs within a given system. As the prognostic capability of a system improves, the concordance index will approach 1, whereas 0.5 represents agreement by chance alone. The original C-index in patients with PMF was 0.573 (95% CI, 0.664-0.582) for DIPSS and 0.587 (95% CI, 0.578-0.596) for MIPSS70. Furthermore, we calculated DIPSS-plus and MIPSS70-plus version 2.0 as well as GIPSS in all patients with PMF with available information on cytogenetic risk according to each model, to evaluate their potential prognostic ability. The C-index was 0.557 (95% CI, 0.546-0.568) for the DIPSS-plus, 0.566 (95% CI, 0.558-0.574) for the MIPSS70-plus version 2.0, and 0.544 (95% CI, 0.532-0.556) for the GIPSS. Collectively, the comparison of all current models developed from nontransplant PMF populations yielded modestly better discrimination when using the DIPSS or the MIPSS70.
Notably, the MYSEC-PM provided moderate performance in patients with post-ET/PV myelofibrosis showing an original C-index of 0.605 (95% CI, 0.593-0.617) while being better than the DIPSS (0.560), which has also recently been used in these patients undergoing stem cell transplantation.
The original C-indices of the MTSS were 0.718 (95% CI, 0.710-0.726) in PMF and 0.701 (95% CI, 0.690-0.711) in post-ET/PV myelofibrosis. Thus, the application of the proposed MTSS indicated overall improvement in discrimination for PMF, as well as post-ET/PV myelofibrosis, with respect to posttransplant outcome. All C-indices are listed in Table 3, and survival curves of existing systems are depicted in supplemental Figure 1, available on the Blood Web site.
Comparison of the performance of prognostic systems in either primary or post-ET/PV myelofibrosis for 5-year survival
| System . | Components . | No. . | C-index (95% CI) . | Bootstrap C-index (95% CI) . |
|---|---|---|---|---|
| Primary myelofibrosis | ||||
| DIPSS | Hemoglobin <10 g/dL, leukocytes >25 × 109/L, circulating blasts ≥1%, age >65 y, constitutional symptoms | 260 | 0.573 (0.664-0.582) | 0.566 (0.557-0.575) |
| DIPSS-plus | DIPSS, transfusion dependence, unfavorable karyotype, platelets <100 × 109/L | 149 | 0.557 (0.546-0.568) | 0.542 (0.531-0.553) |
| MIPSS70 | hemoglobin <10 g/dL, leukocytes >25 × 109/L, platelets <100 × 109/L, circulating blasts ≥2%, fibrosis grade ≥2, constitutional symptoms, absence of CALR type 1-like mutation, HMR category,* ≥2 HMR mutations | 260 | 0.587 (0.578-0.596) | 0.581 (0.572-0.590) |
| MIPSS70-plus version 2.0 | Severity of anemia, circulating blasts ≥2%, constitutional symptoms, absence of CALR type 1-like mutation, HMR category (+U2AF1), ≥2 HMR mutations, 3-tiered cytogenetic risk | 149 | 0.566 (0.558-0.574) | 0.560 (0.551-0.569) |
| GIPSS | Absence of CALR type 1-like mutation; presence of ASXL1, SRSF2, or U2AF1; 3-tiered cytogenetic risk | 149 | 0.544 (0.532-0.556) | 0.532 (0.521-0.543) |
| MTSS | Platelets <150 × 109/L, leukocytes >25 × 109/L, KPS <90%, age ≥57 y, HLA-mismatched unrelated donor, non-CALR/MPL driver mutation genotype, ASXL1 mutation | 260 | 0.718 (0.710-0.726) | 0.710 (0.701-0.719) |
| Post-ET/PV myelofibrosis | ||||
| MYSEC-PM | Hemoglobin <11 g/dL, platelets <150 × 109/L, circulating blasts ≥3%, age, constitutional symptoms, CALR-unmutated genotype | 101 | 0.605 (0.593-0.617) | 0.594 (0.582-0.606) |
| MTSS | 101 | 0.701 (0.690-0.711) | 0.690 (0.679-0.701) |
| System . | Components . | No. . | C-index (95% CI) . | Bootstrap C-index (95% CI) . |
|---|---|---|---|---|
| Primary myelofibrosis | ||||
| DIPSS | Hemoglobin <10 g/dL, leukocytes >25 × 109/L, circulating blasts ≥1%, age >65 y, constitutional symptoms | 260 | 0.573 (0.664-0.582) | 0.566 (0.557-0.575) |
| DIPSS-plus | DIPSS, transfusion dependence, unfavorable karyotype, platelets <100 × 109/L | 149 | 0.557 (0.546-0.568) | 0.542 (0.531-0.553) |
| MIPSS70 | hemoglobin <10 g/dL, leukocytes >25 × 109/L, platelets <100 × 109/L, circulating blasts ≥2%, fibrosis grade ≥2, constitutional symptoms, absence of CALR type 1-like mutation, HMR category,* ≥2 HMR mutations | 260 | 0.587 (0.578-0.596) | 0.581 (0.572-0.590) |
| MIPSS70-plus version 2.0 | Severity of anemia, circulating blasts ≥2%, constitutional symptoms, absence of CALR type 1-like mutation, HMR category (+U2AF1), ≥2 HMR mutations, 3-tiered cytogenetic risk | 149 | 0.566 (0.558-0.574) | 0.560 (0.551-0.569) |
| GIPSS | Absence of CALR type 1-like mutation; presence of ASXL1, SRSF2, or U2AF1; 3-tiered cytogenetic risk | 149 | 0.544 (0.532-0.556) | 0.532 (0.521-0.543) |
| MTSS | Platelets <150 × 109/L, leukocytes >25 × 109/L, KPS <90%, age ≥57 y, HLA-mismatched unrelated donor, non-CALR/MPL driver mutation genotype, ASXL1 mutation | 260 | 0.718 (0.710-0.726) | 0.710 (0.701-0.719) |
| Post-ET/PV myelofibrosis | ||||
| MYSEC-PM | Hemoglobin <11 g/dL, platelets <150 × 109/L, circulating blasts ≥3%, age, constitutional symptoms, CALR-unmutated genotype | 101 | 0.605 (0.593-0.617) | 0.594 (0.582-0.606) |
| MTSS | 101 | 0.701 (0.690-0.711) | 0.690 (0.679-0.701) |
HMR, high-molecular-risk category; MIPSS70, mutation-enhanced International Prognostic Score System for transplantation-age.
High molecular risk category defined as positive for 1 of the mutations: ASXL1, EHZ2, SRSF2, or IDH1/2.
Discussion
Major improvement has been achieved in the understanding of the biology and pathology of myelofibrosis by the discovery of several mutations and their effect on leukemic transformation and survival.7,28-30 The heterogeneity of the disease and the variable outcome can be well determined by specific risk models such as IPSS, DIPSS, or DIPSS-plus.4-6 Most recently, taking the increasing significance of molecular mutation into account, new prognostic models such as the MIPSS70 or the MYSEC-PM specific to transplant-age patients or to post-ET/PV myelofibrosis have integrated molecular mutation to optimize prognostic ability.10,13 These risk models are helpful to determine prognosis in a nontransplant setting but have shown suboptimal results with respect to outcome of allogeneic stem cell transplantation, which is a curative treatment of myelofibrosis but associated with a substantial risk for therapy-related morbidity and mortality.14,15,17,31 One of the reasons might be the lack of patient- and transplant-specific risk factors that influence outcome after allografting independently from disease-specific factors.
Thus, we developed a comprehensive risk model in patients with myelofibrosis who received allogeneic stem cell transplantation aiming to facilitate transplant-specific prognostication and transplant decision making. The resulting MTSS permits integration of clinical, molecular, and transplant-specific risk factors that independently affected survival, enabling 4-level risk stratification, which indicated improvement in prediction of outcome after allogeneic stem cell transplantation. The MTSS may thus facilitate counseling patients with respect to transplantation compared with currently existing models, as well as improve design of clinical trials in the transplant setting.
Using multivariable analysis, we identified pretransplantation thrombocytopenia, leukocytosis, older age, poor performance according to Karnofsky performance status, a non-CALR/MPL or JAK2V617F and triple-negative driver mutation genotype, ASXL1-mutation, and transplantation from an HLA-mismatched unrelated donor as having independent prognostic relevance to survival. Notably, the presence of constitutional symptoms, which is a significant risk factor in all currently existing models, did not contribute to the final model, which may explain different performances in the nontransplant setting compared with the transplant setting. Furthermore, cytogenetic risk stratifications according to DIPSS-plus and MIPSS70-plus version 2.0 showed no effect on survival in univariable analysis. It has been recently shown that allogeneic stem cell transplantation can overcome the negative effect of poor-risk cytogenetics in patients with myelofibrosis.32
It is of interest that clinical variables such as hemoglobin level, transfusion dependence, or number of peripheral blasts that have significant prognostic effect on survival in patients with diagnosed PMF and post-ET/PV did not influence outcome after allogeneic stem cell transplantation significantly, highlighting the importance of distinguishing between risk factors predicting outcome after conventional treatment or allogeneic stem cell transplantation. Regarding molecular genetics, the positive effect of CALR mutation and a negative effect of ASXL1 mutation influencing outcome after diagnosis can also be seen after allogeneic stem cell transplantation and have been recently reported.16,33 Furthermore, our system may support recent reviews suggesting consideration of early transplantation in triple-negative patients and patients with PMF who harbor mutations in ASXL1 in addition to other clinical and transplant-specific risk factors.34 However, the presence of 2 or more high-risk molecular mutations (ASXL1, EZH2, IDH1/2, SRSF2) was not associated with poorer outcome. With respect to other transplant-specific variables, we could not identify a significant effect on outcome in different conditioning intensity, whereas the cytomegalovirus-positive serostatus of the recipient was significant only in the univariable, but not in the multivariable, analysis.
Most recently, a study on 2035 newly diagnosed patients including 309 with myelofibrosis identified distinct genetic subgroups providing a classification of myeloproliferative neoplasms based on causal biologic mechanisms.35 Integrating genomic and clinical data enabled personalized outcome prediction. Although the performance of the model for myelofibrosis with respect to survival showed improvement in comparison with IPSS and DIPSS, differences in performance compared with IPSS remained small, with concordance indices being 0.77 (training cohort) and 0.79 (validation cohort) for the personalized model compared with 0.77 for the IPSS. Because of a lack of information on thrombosis, we could not validate this model in our transplant cohort.
Collectively, our study may help in selecting and counseling patients with myelofibrosis for allogeneic stem cell transplantation in addition to the current available risk scores. Of note, the risk for NRM, especially in the very high risk MTSS group, should always be taken into account and balanced to life expectancy without transplant and other treatment options. Last, we acknowledge several limitations. We cannot exclude the possibility of residual confounding after internal validation, as a result of possible overfitting from variable and threshold selection for these models. However, internal validation with bootstrapping and external validation were used to address these concerns. Another limitation in this study is the lack of information regarding comorbidities. Instead, the Karnofsky performance status was used showing a consistent effect on outcome. The actual performance status of the patient may vary between clinicians or at different times during the transplantation evaluation. Other tools evaluating patient fitness, including the transplantation comorbidity index, also may be used as they become available in large patient data registries.36,37
Despite the limitations identified, our risk model may provide the best prognostic performance for myelofibrosis after transplantation, using readily available clinical, molecular, and transplant-specific data. We show here that this internally and externally validated MTSS accurately discriminated different risk for death and may improve counseling of patients regarding their probable outcome after transplantation in addition to existing models, as well as facilitate design of clinical trials for myelofibrosis undergoing allogeneic stem cell transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.G. and N.K. were responsible for the study design and data analysis and wrote the paper; B.C., R.S., I.T., and A.B. performed laboratory analyses; N.G., M.D., R.B., S.B., M.R., R.S., M.H., and N.K. collected data; and all authors interpreted the data and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolaus Kröger, Department of Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20246 Hamburg, Germany; e-mail: nkroeger@uke.uni-hamburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal