In this issue of Blood, Gagelmann et al1 describe an integrated clinical-molecular prognostic model (Myelofibrosis Transplant Scoring System [MTSS]) to predict outcome post stem cell transplant (SCT) in myelofibrosis (MF). MF is a clonal stem cell neoplasm with heterogeneous clinical phenotypes and well-defined driver mutations found in ∼90% of the cases.2 Despite the introduction of JAK inhibitors,3 MF still remains an incurable disease with a median survival of 4 to 5 years. SCT is an option for MF patients,4 but, because of the very high risk of mortality, the patient selection is very critical.
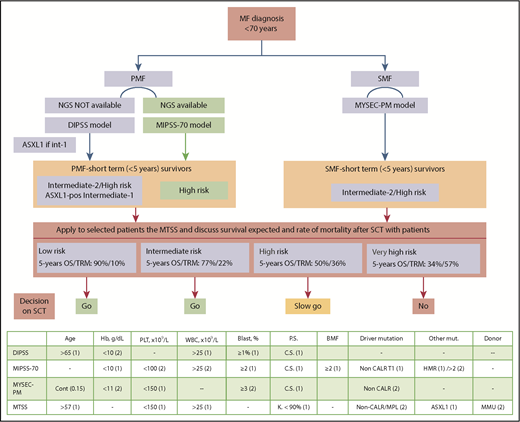
Critical information to personalize management of patients with MF. (Top) Decision flowchart for each patient with MF suitable for SCT. (Bottom) All prognostic models in use with variables and score in parentheses for assessing risk category to each patient. Dynamic International Prognostic Scoring System (DIPSS) categories: low (0); intermediate-1 (1-2), intermediate-2 (3-4), high risk (5-6); Mutation-Enhanced International Prognostic Score System (MIPSS-70) categories: low (0-1); intermediate (2-4), high risk (≥5); Myelofibrosis Secondary Prognostic model (MYSEC-PM) categories: low (<11); intermediate-1 (11-13), intermediate-2 (14-15), high risk (≥16), http://www.mysec-pm.eu; MTSS categories: low (0-2); intermediate (3-4), high (5), very-high risk (6-9). BM, bone marrow; BMF, bone marrow fibrosis; Cont, continuous; C.S., constitutional symptoms; Hb, hemoglobin value; HMR, high-molecular risk; K., Karnofsky; MMU, mismatched unrelated; mut., mutation; PLT, platelet count; P.S., performance status; WBC, white blood cell count.
Critical information to personalize management of patients with MF. (Top) Decision flowchart for each patient with MF suitable for SCT. (Bottom) All prognostic models in use with variables and score in parentheses for assessing risk category to each patient. Dynamic International Prognostic Scoring System (DIPSS) categories: low (0); intermediate-1 (1-2), intermediate-2 (3-4), high risk (5-6); Mutation-Enhanced International Prognostic Score System (MIPSS-70) categories: low (0-1); intermediate (2-4), high risk (≥5); Myelofibrosis Secondary Prognostic model (MYSEC-PM) categories: low (<11); intermediate-1 (11-13), intermediate-2 (14-15), high risk (≥16), http://www.mysec-pm.eu; MTSS categories: low (0-2); intermediate (3-4), high (5), very-high risk (6-9). BM, bone marrow; BMF, bone marrow fibrosis; Cont, continuous; C.S., constitutional symptoms; Hb, hemoglobin value; HMR, high-molecular risk; K., Karnofsky; MMU, mismatched unrelated; mut., mutation; PLT, platelet count; P.S., performance status; WBC, white blood cell count.
A next-generation sequencing (NGS)–based 18-gene panel was available in patients of the study. The transplant regimen was mainly reduced intensity in the training cohort and myeloablative in the validation cohort. Variables included in the model are age ≥57 years, Karnofsky performance status <90%, platelet count <150 × 109/L, leukocyte count >25 × 109/L, HLA-mismatched unrelated donor, presence of the ASXL1 mutation, and a non-CALR/MPL genotype (see figure). The resulting 5-year overall survival (OS) was 90% for low risk, 77% for intermediate risk, 50% for high risk, and 34% for very high risk.
The 5-year survival of the whole cohort was 62%, higher than the 47% reported at the beginning in 1999 by Guardiola et al5 from the European Bone Marrow Transplantation. The results of the study reported here may be impacted by the 9% of patients transplanted with low-risk disease, who would currently not be transplant candidates.6 However, low-risk patients have been included in all SCT reports. Notably, the model is applicable in primary MF (PMF) or in secondary MF (SMF), such as postpolycythemia vera and postessential thrombocythemia MF.
The MTSS will implement current risk models (see figure) with the aim of improving personalization of therapy in MF patients suitable for SCT (age <70 years). Some models include only clinical variables; other models include clinical and molecular, and others are based on karyotypes. Many MF patients have no analyzable metaphases, and about two-thirds of the cases have a normal karyotype,7,8 making karyotype-based models vastly unpracticable. Each patient must receive a prognostic assessment at the time of diagnosis of MF (see figure), according to the MF type: PMF or SMF. For PMF, patients evaluated by NGS can be accessed using the MIPSS-70 model.9 However, if NGS is not available, the DIPSS model still remains a robust and validated tool. In situations of limited NGS availability, at least ASXL1 mutation in intermediate-1 DIPSS-scored young patients should be determined.4 In SMF, the MYSEC-PM10 is recommended because it was specifically developed in SMF. Among MF patients, those with a median expectation of life <5 years are potential candidates for SCT, according to latest European LeukemiaNet recommendations.4 This corresponds to ASXL1 mutated-intermediate-1/intermediate-2/high-risk DIPSS, high-risk MIPSS-70, and intermediate-2/high-risk MYSEC-PM patients (see figure).
At this juncture, MTSS becomes helpful. Low- and intermediate-risk MTSS has a clear indication for SCT, as the 5-year OS of 90% and 77%, respectively, is superior to other therapies, and this result balances the rate of nonrelapse mortality (NRM) of 10% to 22%. The following cases illustrate the difficulties in the management of high-risk and very-high-risk MTSS situations.
Case 1: A 68-year-old man with post polycythemia vera–MF (intermediate-2 MYSEC-PM). The projected life expectancy is 4.5 years (MYSEC-PM); hence, he is an SCT candidate. However, his MTSS category is high risk, resulting in a 5-year median survival of 50% and a transplant related mortality (TRM) of 36%. In this case, survival is equivalent with standard therapy or proceeding to SCT. It seems reasonable not to proceed to SCT. However, if the patient were younger (aged 40-60 years), it would be reasonable to proceed to SCT as the only therapy, with a survival plateau after 5 years.
Case 2: A 60-year-old woman with PMF (NGS data available) with high-risk MIPSS-70 and an estimated 5-year survival of 29%. This patient has an MTSS score of 7, a very-high risk MTSS category. Her 5-year survival after SCT is 34%, with NRM of 57%. As survival is poor with either option, a clinical trial of novel therapy should be pursued.
In conclusion, prognostic models are of critical importance to personalize management of MF. Currently, assessment of patients should include molecular evaluation with NGS or, at least, evaluation of ASXL1 mutation. SCT still remains a high-risk procedure in MF, but, after 5 years, 40% of the patients can be considered cured. The MTSS helps in individualizing the SCT decision in MF patients.
Conflict-of-interest disclosure: F.P. declares no competing financial interests.