Abstract
The niche that supports hematopoietic stem and progenitor cells (HSPCs) in the bone marrow is a highly dynamic structure. It maintains core properties of HSPCs in the steady state, and modulates their proliferation and differentiation in response to changing physiological demands or pathological insults. The dynamic and environment-sensing properties of the niche are shared by the innate immune system. Thus, it is not surprising that innate immune cells, including macrophages and neutrophils, are now recognized as important regulators of the hematopoietic niche and, ultimately, of the stem cells from which they derive. This review synthesizes emerging concepts on niche regulation by immune cells, with a particular emphasis on neutrophils. We argue that the unique developmental, circadian, and migratory properties of neutrophils underlie their critical contributions as regulators of the hematopoietic niche.
Introduction
Neutrophils are innate, polymorphonuclear leukocytes that act as the first line of host defense against invading pathogens. Central to their function is their ability to be recruited to sites of infection, to recognize and phagocytose microbes, and to kill pathogens through a combination of cytotoxic mechanisms (reviewed in Mayadas et al1 ). These include the production of reactive oxygen species (ROS), the release of antimicrobial peptides, and the extrusion of their nuclear contents to form neutrophil extracellular traps. Beyond their prominent immune roles, recent years have seen a remarkable emergence of unexpected nonimmune functions of neutrophils in homeostasis as well as in diseases with an important inflammatory component, including systemic lupus and cancer.2
A wealth of recent studies have begun to dissect the function of immune cells, including neutrophils, in the bone marrow. These studies most prominently highlight the diversity of properties of a cell type that not long ago was regarded as purely cytotoxic and proinflammatory. Here, we review fundamental aspects of neutrophil and bone marrow niche biology, and discuss the functional interplay between neutrophils and other immune cells within these niches that help to preserve hematopoietic stem and progenitor cells (HSPCs). We finally consider temporal regulation of the hematopoietic niche driven in part by the unique circadian properties of neutrophils, as this highlights novel layers of interaction between immunity and hematopoiesis.
Developing neutrophils and neutrophils in development
Neutrophils are short-lived cells, as they are generally believed to circulate for only 6 to 12 hours in mice and humans.3,4 Their short lifespan in circulation demands constant production and release from the bone marrow, with an estimated production rate in humans of ∼1010 cells per day.5 Given their indispensable antimicrobial roles but potential toxic activity in tissues, both excessive and deficient production of neutrophils can have major detrimental consequences for the organism. Indeed, neutrophil homeostasis is tightly regulated through a balance between granulopoiesis, storage, and egress from the bone marrow, intravascular margination, clearance, constitutive death by apoptosis,6 and elimination through phagocytosis in specific organs.5,7
Neutrophils are formed within the bone marrow through a series of progressively differentiated precursors in a process termed granulopoiesis. The most immature long-term or short-term stem cells give rise to multipotent progenitors, common myeloid progenitors, and granulocyte-macrophage progenitors (GMPs). Only recently, GMPs have been shown to produce neutrophil-committed proliferative precursors (NeP and pre-Neu) that differentiate into nonproliferative immature neutrophils, and give rise to the mature neutrophils that are released into the bloodstream8,9 (Figure 1).
Functional and phenotypic diversity of neutrophils in the bone marrow. Neutrophils are produced inside of the bone marrow (BM) through progressive maturation of hematopoietic progenitors (long-term hematopoietic stem cells [LT-HSCs] to GMPs). Proliferative precursors (NeP and preNeu) differentiate into immature neutrophils and finally into mature neutrophils that are released into blood. A fraction of aged neutrophils return into the marrow after several hours in the circulation. Top and bottom panels indicate specific phenotypes and functions, respectively, of neutrophils at each stage of their life cycle. HSC, hematopoietic stem cell; HSCT, hematopoietic stem cell transplantation; HSPC, hematopoietic stem and progenitor cell; MPP, multipotent progenitor; ST, short-term; TNF, tumor necrosis factor. Professional illustration by Patrick Lane, ScEYEnce Studios.
Functional and phenotypic diversity of neutrophils in the bone marrow. Neutrophils are produced inside of the bone marrow (BM) through progressive maturation of hematopoietic progenitors (long-term hematopoietic stem cells [LT-HSCs] to GMPs). Proliferative precursors (NeP and preNeu) differentiate into immature neutrophils and finally into mature neutrophils that are released into blood. A fraction of aged neutrophils return into the marrow after several hours in the circulation. Top and bottom panels indicate specific phenotypes and functions, respectively, of neutrophils at each stage of their life cycle. HSC, hematopoietic stem cell; HSCT, hematopoietic stem cell transplantation; HSPC, hematopoietic stem and progenitor cell; MPP, multipotent progenitor; ST, short-term; TNF, tumor necrosis factor. Professional illustration by Patrick Lane, ScEYEnce Studios.
The ultimate elimination of neutrophils is as important as their production, and these 2 processes must be tightly coordinated to maintain a constant supply and steady number of neutrophils in blood.10 This is important because overproduction of neutrophils can aggravate cytotoxic damage in healthy tissues as seen in many inflammatory diseases, whereas neutropenia inevitably results in recurrent infections and, paradoxically, chronic inflammatory states.11 A key mechanism regulating neutrophil homeostasis was reported in a seminal study by Ley and colleagues, and involves the interleukin 23 (IL-23)/IL-17/granulocyte colony-stimulating factor (G-CSF) feedback circuit.12 Senescent neutrophils that migrate to peripheral tissues are phagocytosed by tissue-resident phagocytes, including macrophages and dendritic cells,12 in a process that relies, at least partially, on the liver X receptors (LXRs).13 Activation of LXRs in engulfing phagocytes inhibits transcription of Il23, a cytokine that boosts granulopoiesis by promoting the production of IL-17, which in turn induces the production by stromal cells of G-CSF, the main granulopoietic factor.13 This homeostatic loop becomes evident in mice deficient in adhesion molecules, in which neutrophils have impeded egress from blood into tissues and consequent reduced uptake by tissue phagocytes, leading to unleashed production of IL-23 and IL-17, and therefore supraphysiological levels of G-CSF that drive the overproduction and release of neutrophils into blood.12 This study was important not only for identifying a mechanism for homeostatic regulation of neutrophil numbers, but also for providing the first link between neutrophils and functional regulation of hematopoiesis. The receptor CXCR2 is not only needed for the normal release of neutrophils from the bone marrow into blood, but also for their migration into tissues. Deficiency in Cxcr2 or its ligand CXCL5 produced by intestinal cells also results in dysregulation of the IL-17/G-CSF axis and microbiota composition, resulting in elevated medullary granulopoiesis and neutrophilia.12,14 Interestingly, studies in antibiotic-treated mice demonstrated a reciprocal regulation, whereby the microbiota are an important innate stimulus for IL-17–producing cells in the intestine and G-CSF production, thereby participating in neutrophil production and immune competence of the organism.15
The cross talk between mature immune cells and hematopoietic stem cells (HSCs) is already evident from embryonic life, a stage at which specific populations of primitive immune cells have an essential role in determining HSC fate. For instance, yolk sac–derived macrophages that migrate to the fetal liver around embryonic day 10.5 contribute substantially to the first wave of hematopoiesis.16 The fetal liver serves as the main hematopoietic organ during embryonic development until HSCs move to the bone marrow, which becomes the primary site of hematopoiesis from the perinatal period onward. It is striking that yolk sac–derived macrophages persist in functionally distinct tissues in adulthood such as in brain (microglia), epidermis (Langerhans cells), and lung (alveolar macrophages),16 among many other tissues, implying that early dissemination of immune cells is important for prenatal and adult life in hematopoietic and nonhematopoietic organs. Also during embryonic life, a subset of primitive neutrophils that lies in the dorsal aorta of the zebrafish embryo was shown to play an important role in determining HSC fate.17 These cells were shown to be the main source of tumor necrosis factor α (TNFα), a cytokine needed for the emergence and specification of HSCs in the embryo, thereby providing an example of early immune-driven determination of HSC fate in development.17
The hematopoietic bone marrow niche
HSPCs proliferate and differentiate in a highly regulated manner, thus giving rise to all immune subsets in the bone marrow, or after migrating into extramedullary hematopoietic or lymphoid organs. Regulation of hematopoiesis requires a highly dynamic and tightly regulated orchestration of stem cell–intrinsic programs.18 Notably, the realization that HSPCs lost repopulating ability when placed outside of the marrow led to the formulation of the “niche” concept, which proposed a specific stem cell–supportive environment inside of the medullary space.19,20 This supportive niche is composed of a plethora of cellular components, which regulate HSPC activity by supplying growth regulators and retention factors. The specific location of HSCs in the vast medullary space has been controversial (reviewed in Wei and Frenette21 ); many studies pointed to localization close to the endosteal region,22-25 whereas others studies suggested that HSCs localized randomly in the bone marrow or were perisinusoidal.26 It has become increasingly clear that the vast majority of HSCs in the marrow localize adjacent to blood vessels, therefore proximal to perivascular cells. Endothelial cells are key sources of CXCL12 and the cytokine stem cell factor (SCF) that maintain HSPCs.27-30 In addition to the endothelium, rare populations of perivascular cells are also key sources of CXCL12 and SCF. Aided by the use of multiparametric imaging with different markers and lineage-specific reporter genes, the complex heterogeneity that exists among stromal cells is now being clarified. Based on the brightness and morphology of Nestin–green fluorescent protein-positive (GFP+) cells, 2 subsets of mesenchymal progenitor cells were identified.24 Nes-GFP-bright cells are scarce and associate with arterioles, whereas the GFP-dim cells are more abundant, reticular shaped, and associate with sinusoids. Interestingly, quiescent HSCs preferentially localize near arteriolar cells. Nes-GFP-bright cells express the pericyte markers NG2+ and α-smooth muscle actin and produce abundant CXCL12 needed for HSC localization and quiescence.31 In contrast, Nes-GFP-dim cells, which can be also identified by expression of the leptin receptor (LepR+), are an important source of SCF that help maintain constant numbers of HSCs in the bone marrow.31
Besides cells of mesenchymal origin, early findings provided strong evidence that the nervous system also regulates the hematopoietic niche and HSPC properties.32-34 Sympathetic nerves that align with the medullary vasculature regulate the expression of stromal CXCL12 and thereby the traffic of HSPCs in and out of the bone marrow under homeostasis or stress conditions.34-36 Specifically, release of the neurotransmitter noradrenaline by sympathetic nerves signals stromal cells through the β3-adrenergic receptor, leading to rapid downregulation of Cxcl12 expression. Interestingly, studies showed that noradrenaline secretion follows a circadian pattern controlled by the core genes of the molecular clock, which elegantly explained the diurnal release of HSPCs into blood.34 Glial fibrillary acidic protein (GFAP+) nonmyelinating Schwann cells that ensheath sympathetic nerves are also functional regulators of HSC proliferation by providing active transforming growth factor β1 (TGFβ1).37
Niche regulation by hematopoietic descendants
In addition to stromal niche components and sympathetic nerves, a growing list of hematopoietic cells that descend from HSPCs have been shown to influence HSC homeostasis and fate, including macrophages, megakaryocytes (MKs), regulatory T cells (Tregs), and neutrophils. Bone marrow–resident macrophages were the first among this progeny shown to favor retention of HSPCs by reinforcing the function of Nestin+ cells and osteoblasts,38-40 an effect that opposes the niche-inhibiting and mobilizing effects of the sympathetic nervous system. Experiments in which CD169+ macrophages were acutely depleted demonstrated that their elimination was sufficient to induce HSPC egress into the bloodstream.38 Interestingly, macrophages regulate HSPCs also under stress; in a transplantation setting, radiation eliminates the majority of leukocytes but spares a population of resident macrophages that repopulate the spleen and marrow via autonomous cell division.41 These CD169+ radiation-resistant macrophages are needed for optimal donor-derived HSC reconstitution.41
MKs, the precursors of platelets, are in close contact with sinusoidal vessels in the marrow, where they extend cytoplasmic protrusions into the vessel lumen to release newly produced platelets. A subset of HSCs localizes near MKs in the sinusoids, and this spatial relationship was shown by several studies to correlate with regulation of HSC pool size.42,43 Specifically, HSPCs expanded dramatically after depletion of MK in Cxcl4-Cre;iDTR mice. These effects could be pinned down to the production of key regulators of HSPC proliferation by MKs, including CXCL4 and TGFβ1, both of which promote HSC quiescence. Consequently, deletion of these factors from MKs resulted in increased HSC numbers in the steady state. In contrast to these results, a separate study showed that depletion of MKs resulted in reduction of HSC numbers despite a similar loss of quiescence, an effect that was accounted for by the production of thrombopoietin.44 Besides homeostasis, MKs can promote HSPC recovery after ablation with irradiation by secretion of fibroblast growth factor 143 or indirectly through osteoblast expansion.45
The bone marrow is a major reservoir of a population of CD4+CD25+ T lymphocytes with immune-modulatory functions or Tregs.46 A subset of Tregs that expresses high levels of the stem marker CD150 was found in the endosteal region of the bone marrow, proximal to HSPCs.47,48 CD150high Tregs control HSPC quiescence and engraftment through the production of adenosine generated via the CD39 ectoenzyme,48 and it has been proposed that Tregs confer immune privilege to the HSPC niche.47
In summary, ample evidence now shows that the hematopoietic niche is regulated, secured, and nurtured by the very descendants of HSPCs residing therein, perhaps providing a regulatory loop that feeds on output cells and benefits from the exquisite sensing properties of mature immune cells. Given the precedents described earlier in this section, it is not surprising that other hematopoietic cell lineages can actively regulate the bone marrow niche. In the next section, we focus our discussion on neutrophils, the most abundant among HSPC descendants, whose extreme sensitivity to stress, tissue damage, and even temporal cues may provide additional layers of regulation of the bone marrow niche.
Regulation of HSPC quiescence and proliferation by neutrophils
Besides perivascular cells and MKs, myeloid cells have been shown to maintain HSPC quiescence through a negative feedback histaminergic circuit. Indeed, a myeloid population expressing the histidine decarboxylase produces histamine (Figure 1). This biogenic amine inhibits active cycling of a myeloid-biased histidine decarboxylase–high HSC population through the histamine receptor 2, and promotes its self-renewal.49 This pathway elicited by granulocytes and possibly other myeloid subsets was important for HSPC maintenance because ablation of histamine-producing cells caused myeloid-biased HSCs and progenitors to exit dormancy and induced loss of serial transplantation capacity.49
Along the same line, neutrophils stimulate emergency myelopoiesis via production of ROS, which oxidizes the phosphatase and tensin homolog phosphatase to directly activate HSPC proliferation upon acute infection or inflammation.50 We expect that, as we continue to extend our knowledge on neutrophil biology in the bone marrow, new mechanisms by which these cells directly regulate HSPC fate will emerge. At present, however, the most prominent known roles of neutrophils on HSPCs are mediated through regulation of their niche, as discussed next.
Role of neutrophils in regeneration of the bone marrow niche
HSC transplantation (HSCT) remains the only curative treatment of most malignant and nonmalignant hematopoietic diseases. In this procedure, the diseased host hematopoietic cells are wiped out by high-dose chemotherapy or radiotherapy. Healthy HSPCs and more mature hematopoietic cells are then transferred into the recipient’s circulation where they home to the bone marrow to engraft and regenerate a new hematopoietic system. Unfortunately, the treatments used to eliminate the host hematopoietic cells invariably cause an almost complete destruction of the vascular HSPC niche in the bone marrow. Specifically, they ablate the sinusoidal vasculature and associated perivascular cells, while leaving arteries and arterioles mostly intact.51-53 Although the transplanted HSPCs can engraft for short periods near endosteal arterioles and MKs,43,45 long-term restoration of normal hematopoiesis demands reestablishment of a healthy sinusoidal network,51-53 as initially demonstrated by Rafii and colleagues.52 Indeed, deletion of vascular-borne vascular endothelial growth factor receptor 2 does not affect baseline hematopoiesis, but strongly impairs regeneration of the vasculature and the hematopoietic compartment after injury.52 In addition to the aforementioned functions of supporting nutrients and providing a niche for HSPCs, the sinusoidal network also produces many molecules like Notch ligands and pleiotrophin that promote HSPC engraftment specifically after injury.51,54-57 Thus, regeneration of the sinusoidal network is the rate-limiting step in restoring healthy hematopoiesis after HSCT, and a long-standing question has been which environmental cues instruct vascular regeneration of the damaged niche.
We recently discovered that bone marrow Gr1+CD115− neutrophils drive sinusoidal regeneration after transplantation.58 We noticed that, in mice transplanted with total bone marrow mononuclear cells, regeneration of the host vascular niche correlated directly with the number of donor hematopoietic cells transplanted, and adoptive transfer experiments demonstrated that only bone marrow neutrophils were capable of driving sinusoidal regeneration. In agreement, depletion of mature neutrophils from the initial graft or genetic ablation of donor-derived neutrophils delayed regeneration of the vasculature. These experiments indicated that neutrophils are both necessary and sufficient to drive vascular regeneration after HSCT. Imaging experiments showed that bone marrow neutrophils are selectively recruited to the injured sinusoids, where they secrete TNFα, a cytokine that promoted endothelial cell survival and regeneration of the sinusoids58 (Figure 2). After transplantation, donor HSPCs initiate a proregenerative program that greatly increases their proliferation and their capacity to generate neutrophils and other myeloid cells.59 These findings suggested that newly generated neutrophils can promote regeneration of the sinusoidal network, which in turn facilitates hematopoietic progenitor engraftment.58 This positive feedback loop continues until the sinusoidal network is restored and the bone marrow returns to homeostasis. Surprisingly, the signals and mechanisms that sense regeneration of the sinusoidal niche, halt further vessel growth, and induce HSPC return to quiescence are almost completely unknown, although it is likely that TGFβ signaling plays a major role in this process.53,60 Identification of these mechanisms may lead to the development of better therapies to promote faster myeloid cell recovery, with restoration of innate immunity and reduced infections after HSCT.
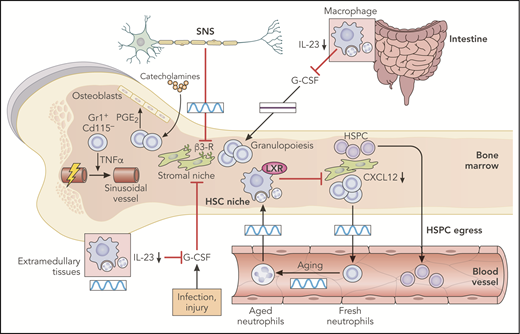
Regulation of the hematopoietic bone marrow niche. The sympathetic nervous system (SNS) exerts control on the HSC niche by the circadian release of catecholamine, which targets β3-adrenergic receptors on stroma cells. The same signals can act through neutrophils to produce prostaglandin E2 (PGE2) and stimulate the osteoblastic niche. The stromal niche is also circadianally regulated by aged neutrophils that return to the bone marrow after only several hours in the circulation. Aged neutrophils that infiltrate the bone marrow are engulfed by macrophages and activation of the LXRs lead to inhibition of the hematopoietic niche. Excessive G-CSF production associated with several inflammatory processes or impaired neutrophil clearance in extramedullary tissues is also a potent inhibitor of the HSPC niche. All of these regulatory mechanisms ultimately inhibit production of CXCL12, thereby promoting HSPC egress into blood. This has been shown in the intestine, where neutrophil infiltration in the mucosa and engulfment of neutrophils by tissue-resident macrophages inhibits the IL-23/IL-17/G-CSF axis and remotely supports niche activity in a circadian-independent manner. Boxes indicate the presence or absence of circadian oscillations in each tissue. Professional illustration by Patrick Lane, ScEYEnce Studios.
Regulation of the hematopoietic bone marrow niche. The sympathetic nervous system (SNS) exerts control on the HSC niche by the circadian release of catecholamine, which targets β3-adrenergic receptors on stroma cells. The same signals can act through neutrophils to produce prostaglandin E2 (PGE2) and stimulate the osteoblastic niche. The stromal niche is also circadianally regulated by aged neutrophils that return to the bone marrow after only several hours in the circulation. Aged neutrophils that infiltrate the bone marrow are engulfed by macrophages and activation of the LXRs lead to inhibition of the hematopoietic niche. Excessive G-CSF production associated with several inflammatory processes or impaired neutrophil clearance in extramedullary tissues is also a potent inhibitor of the HSPC niche. All of these regulatory mechanisms ultimately inhibit production of CXCL12, thereby promoting HSPC egress into blood. This has been shown in the intestine, where neutrophil infiltration in the mucosa and engulfment of neutrophils by tissue-resident macrophages inhibits the IL-23/IL-17/G-CSF axis and remotely supports niche activity in a circadian-independent manner. Boxes indicate the presence or absence of circadian oscillations in each tissue. Professional illustration by Patrick Lane, ScEYEnce Studios.
Although the role of neutrophils in bone marrow regeneration was previously unclear, it was well established that they contributed to tissue regeneration (reviewed in Wang61 ). Neutrophils are recruited to injured tissues via damage-associated molecular patterns,62 where they can exert both positive and negative effects in the regeneration program. This is dependent on cellular context and in the amount and type of neutrophils recruited to each tissue.61,63 In the context of vascular development and repair, it is now clear that different neutrophil subsets cross talk with endothelial cells to regulate their function.
As described earlier in “Developing neutrophils and neutrophils in development,” embryonic neutrophils induce generation of definitive HSCs by signaling via TNFα to the hemogenic endothelium.17 The Phillipson group identified a vascular endothelial growth factor receptor 1–positive neutrophil subset in the circulation (representing ∼5% of blood neutrophils) that is selectively recruited to hypoxic tissues, where they induce vessel growth via matrix metallopeptidase 9 release.64-66 Intriguingly, however, blood-borne neutrophils are unable to induce vascular regeneration in the marrow despite expressing high amounts of TNFα, a limitation that may reflect their inability to home to injured sinusoids after adoptive transfer.58 An emerging concept is that neutrophils are a heterogeneous population both in tissues and in peripheral blood, and that they can adopt unique physiological functions.67,68 In the particular case of medullary regeneration, we highlight that there are at least 2 subsets of angiogenic neutrophils: 1 in the bone marrow that acts on niche-associated sinusoids and 1 in the periphery that acts on peripheral vessels. A recent study by the Ng group also showed that classically defined bone marrow Gr1+CD115− neutrophils are in fact a heterogeneous population that comprises a proliferating neutrophil progenitor as well as immature and mature neutrophils with transcriptional signatures distinct from those of circulating neutrophils.8 It will be interesting to dissect the behavior of each of these medullary neutrophil subsets after HSCT and their contribution to vascular niche regeneration.
Bone marrow neutrophils are recruited specifically to injured sinusoids. This direct interaction is clearly important for the sinusoids as areas of the bone marrow that have no neutrophils showed no sinusoidal regeneration.58 In the steady state, neutrophil trafficking is regulated, almost exclusively, via CXCR2 and CXCR4.69 However, pharmacological blockade of both pathways does not affect neutrophil recruitment to injured vessels,58 thereby indicating the existence of an unidentified mechanism in the sinusoids, induced by damage to the vasculature that specifically recruits neutrophils to injured bone marrow vessels.
In addition to aiding regeneration of the vascular niche, neutrophils have been reported to support niche activity by enhancing the capacity of preosteoblastic cells to produce osteopontin, an important retention factor for HSPCs in the marrow.70 Interestingly, adrenergic stimulation of neutrophils through the β3 receptor induced production of prostaglandin E2, a well-known support factor for hematopoiesis,71 which in turn induced osteoblastic activity through the EP4 receptor.70 Thus, neutrophils appear to counteract, to some extent, the inhibitory effects that catecholamines exert on the niche, thereby preventing excessive HSPC mobilization (Figure 2). The identification of neutrophils as intermediary and regulators of the mobilization process provides important mechanistic links between the various pathways that regulate hematopoietic niches.
Circadian regulation of the hematopoietic niche
In almost all life forms on Earth, the planet’s rotation has led to the evolution of daily circadian cycles of 24 hours. In mammals, peripheral clocks are normally synchronized with the environment by entrainment from daily exposure to light-dark cycles. The central circadian pacemaker located in the suprachiasmatic nuclei receives photic information conducted from the retina. The synchrony between autonomous circadian clocks found in all major organs and tissues is maintained by a complex network, involving neuronal signaling, secretion of hormones, and metabolic cues (reviewed in Scheiermann et al72 ). As discussed in “The hematopoietic bone marrow niche,” the bone marrow is extensively innervated by autonomic nerve fibers, including sympathetic nerves, which play important physiological roles in the bone marrow. Sympathetic nerves have been shown to be responsible for cytokine-elicited mobilization of HSPCs outside of the bone marrow into blood,32 although active signaling in monocytic cells has also been demonstrated.39 G-CSF, a cytokine broadly used in the clinic to mobilize HSPCs into circulation for transplantation therapies, promotes the release of noradrenaline by autonomic neurons located in the periphery. Released adrenaline mediates the suppression of osteoblasts located in the endosteal marrow, thereby reducing the synthesis of CXCL12 and causing HSPC mobilization.32 Additionally, sympathetic nerves regulate perivascular Nestin-GFP+ stem cells by acting on β3 adrenergic receptors.35 This neural-mesenchymal axis is responsible for the circadian expression of CXCL12 by bone marrow stromal cells, which causes the homeostatic release of HSPCs into circulation.34 In mice, the lowest levels of CXCL12 protein in the medullary space coincide with HSPC egress around zeitgeber time 5 (ZT5, or 5 hours after the onset of light), and the highest CXCL12 levels occur at ZT13 and correlate with the lowest numbers of circulating HSPCs.34 In contrast to mice, humans display inverted circadian oscillations with maximum levels of progenitors in blood in the evening.73
Given the bidirectional flux between blood and marrow, it is not surprising that adrenergic nerves also control the expression of endothelial-adhesion molecules in the medullary vasculature, as these adhesion molecules are necessary for HSPC homing back to the marrow.74 It is also likely that a cross talk exists between the levels of the chemokine CXCL12 and those of endothelial-adhesion molecules; for instance, in mice, higher levels of CXCL12 at night correlate with a higher retention of HSCs in the bone marrow. The genuine circadian nature of this process was illustrated by jet-lag experiments showing that repeated shifts in light cycle were sufficient to ablate circadian HSPC recruitment into tissues.74
Interestingly, much like HSPCs, mature leukocytes infiltrate the bone marrow in a circadian manner,74 with peak homing to the marrow and other organs at ZT13 in mice. The circadian migration of mature leukocytes (and HSPCs) may be beneficial to provide a readily available set of tissue-resident leukocytes that mediate immune defense during the animal’s active phase, when the individual’s probability of injury or encountering pathogens is highest. The circadian fluxes of mature leukocytes that return to the marrow also suggest potential regulation of bone marrow niches by cells that have “sampled” the extramedullary environment. For example, it is likely that various myeloid cell subsets regulate circadian oscillations in HSPC activity through TNFα. Indeed, this cytokine has been shown to regulate circadian migration, proliferation, and differentiation through modulation of ROS and melatonin signaling in HSPCs, and its medullary levels are controlled in part by neutrophils and monocytes.58,75
Neutrophil aging and temporal control of the hematopoietic niche
Neutrophils are the most abundant myeloid population in the bone marrow. Because of the short lifespan, vast amounts of neutrophils must be released into the blood every day to maintain homeostatic numbers.10 This implies that, even under homeostatic conditions, large numbers must also be eliminated every day, yet possible functions for these naturally cleared neutrophils were enigmatic.76,77
Only recently, we and others discovered that circulating neutrophils undergo circadian fluctuations that affect not only neutrophil numbers, but also their phenotype. This spontaneous change over time is referred to as neutrophil aging.78 As neutrophils age in the circulation, their repertoire of surface receptors change: they upregulate markers like CXCR4 and very-late-activation antigen 4, both of which are important for the retention in and homing to the marrow,76,77,79 and downregulate others including CD62L (L-selectin) and CXCR277,80 (Figure 1). This CXCR4hi CD62Llo population of aged neutrophils follows marked circadian oscillations throughout the day and is completely cleared out from circulation by night (ZT13), when the active behavioral phase of the mice begins.77 Interestingly, recent studies in mice have proposed that aged neutrophils gain immune competence by enhancing β2 integrin–dependent adhesion, as well as their capacity to phagocytose and to form DNA-based neutrophil extracellular traps.81,82 In addition, microbiota-derived metabolites have been proposed to drive neutrophil aging through Toll-like receptor signaling,82 although our own data suggest that cell-intrinsic circadian programs can also drive aging.80 Thus, although the evolutionary drive and physiological role of this diurnal aging process of neutrophils remains to be fully elucidated, it is tempting to speculate that diurnal “priming” of neutrophils is needed for these cells to fully mature and to fulfill additional functions after their lifetime in blood, once they have cleared into tissues.
Aged neutrophils that clear from the circulation into tissues are believed to be ultimately engulfed and eliminated by tissue macrophages.78,83 The bone marrow is one of the tissues in which aged neutrophils are cleared in larger numbers. Clearance in this organ not only serves to control neutrophil numbers but also, importantly, generates homeostatic signals that modulate the bone marrow niche.77 When aged neutrophils infiltrate the mouse bone marrow between ZT5 and ZT13, they are engulfed by tissue-resident macrophages. This efferocytic process generates LXR-dependent, but otherwise undefined, signals that downregulate the number of niche cells and, consequently, the amount of CXCL12 in the marrow, thereby promoting HSPC egress into blood (Figure 2). The numbers of CXCL12-producing reticular cells and osteoblasts in the bone marrow consistently increase when neutrophils are experimentally depleted, indicating that neutrophils modulate the size of the niche stroma. More importantly, interruption of this natural niche-inhibitory pathway by depletion of circulating neutrophils or macrophages completely blunted the diurnal oscillations of HSPCs in blood, indicating that circadian clearance of aged neutrophils drives rhythms in the hematopoietic niche.77 These findings in mice reveal a coalescence of hematopoietic, neural, and immune inputs in the bone marrow to provide multilayered regulation of hematopoiesis. Importantly, alterations of this axis appear to powerfully influence disease, as shown in the context of cardiovascular disease or cancer.84-86
Contrary to common belief, homeostatic clearance of aged neutrophils is not unique to the bone marrow, spleen, or liver: it also takes place in many extramedullary tissues such as the lung, skin, or muscle in which they can perform tissue-specific roles.2,78,87 Surprisingly, infiltration of neutrophils in the intestinal mucosa enhances bone marrow niche activity remotely, by preventing Il23 transcription in intestinal macrophages. Similar to the aforementioned “neutrostat” model,12 inhibition of this cytokine results in reduced systemic levels of G-CSF and preserved niche function, thereby preventing excessive mobilization of HSPCs into blood. However, unlike the rhythmic inhibitory roles of marrow-infiltrating neutrophils,77 infiltration in the intestine does not follow circadian patterns and, consequently, niche regulation from the intestine does not influence the diurnal oscillations of circulating HSPCs87 (Figure 2). These recent findings expand the regulatory mechanisms of hematopoietic niches to include distant anatomical sites, and are consistent with the reported effects of microbiota-derived signals emanating from the gut in regulating stem cell and niche activity.88-90
Concluding remarks
It is becoming increasingly clear that dysregulation of hematopoiesis is an important underlying driver of disease. Therefore, understanding the multiple regulatory mechanisms of this highly dynamic process becomes a question of major biomedical relevance. Disturbance of neural regulation of the niche occurs during organismal aging91 and is also prominent in the context of ischemic disease,92,93 whereas dysregulated cytokine pathways appear to be more common in cancer.84 We have discussed here emerging evidence that neutrophils provide additional layers of regulation in hematopoiesis. The realization that neutrophils influence multiple aspects of niche physiology, from maintenance of the mesenchymal niche to HSPC quiescence, demands urgent evaluation of their contribution to inflammatory disease and hematological malignancies. Neutrophils are also instrumental in regenerating the injured vascular niche and may provide strategies to accelerate regeneration of patients undergoing HSCT. More generally, we propose that the unique temporal properties of neutrophils, their basal presence in multiple tissues, and exquisite capacity to sense danger make these cells ideal intermediaries for niche regulation and repair not only in the bone marrow, but also in other tissues that demand rapid responses to environmental challenges.
Acknowledgments
This work was supported in part by SAF2015-65607-R and Fondo Europeo de Desarrollo Regional (FEDER) (A.H.); Ministerio de Ciencia, Innovacion y Universidades (MCIU) for fellowship BES-2014-068915 (I.C.); and R01 HL136529-01 from the National Institutes of Health, National Heart, Lung, and Blood Institute (D.L.). The Centro Nacional de Investigaciones Cardiovasculares (CNIC) was supported by the MCIU and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MCIU award SEV-2015-0505).
Authorship
Contribution: I.C., D.L., and A.H. wrote this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrés Hidalgo, Area of Cell and Developmental Biology, Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III, Madrid 28029, Spain; e-mail: ahidalgo@cnic.es.
REFERENCES
Author notes
I.C., D.L., and A.H. contributed equally to the writing of this review article.

![Figure 1. Functional and phenotypic diversity of neutrophils in the bone marrow. Neutrophils are produced inside of the bone marrow (BM) through progressive maturation of hematopoietic progenitors (long-term hematopoietic stem cells [LT-HSCs] to GMPs). Proliferative precursors (NeP and preNeu) differentiate into immature neutrophils and finally into mature neutrophils that are released into blood. A fraction of aged neutrophils return into the marrow after several hours in the circulation. Top and bottom panels indicate specific phenotypes and functions, respectively, of neutrophils at each stage of their life cycle. HSC, hematopoietic stem cell; HSCT, hematopoietic stem cell transplantation; HSPC, hematopoietic stem and progenitor cell; MPP, multipotent progenitor; ST, short-term; TNF, tumor necrosis factor. Professional illustration by Patrick Lane, ScEYEnce Studios.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/20/10.1182_blood-2018-10-844571/3/m_blood844571f1.png?Expires=1769103749&Signature=nlw160GmmebjoYFiSPkXWvn1FnBytRn8mYeJ4SyBfLZs0-FiX~aqRS8gFR71ZteOyzQIPRqm13uFjHEoQTVQy8EVDpBN2Ehnoz8BscmY9NN3RQJuiAuBFxe-Lp8FRjrGHF6rrab1boimHCT34U~-wANQLiHAowE1BfNBbBCA7~STulxCYMbZAsVNN1ihwKlXcMjm2cLnHR4Heue-x6bsJKpoIzFBjjV~idNQ-IcBKtpOefCOgQ5WzV9YSa2FTfP~zw-gq2O1zeda4y-070AcPdhyQpzO9cuRNxqs3V9GTFMUTPnzvpSuqH5vaGXz9QVIDX~02hK23ar~OqD0VTTsRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal