In this issue of Blood, Hirayama and colleagues report that a favorable cytokine profile induced by lymphodepletion is associated with durable remissions in patients with aggressive non-Hodgkin lymphoma treated with anti-CD19 chimeric antigen receptor (CAR) T-cell therapy.1
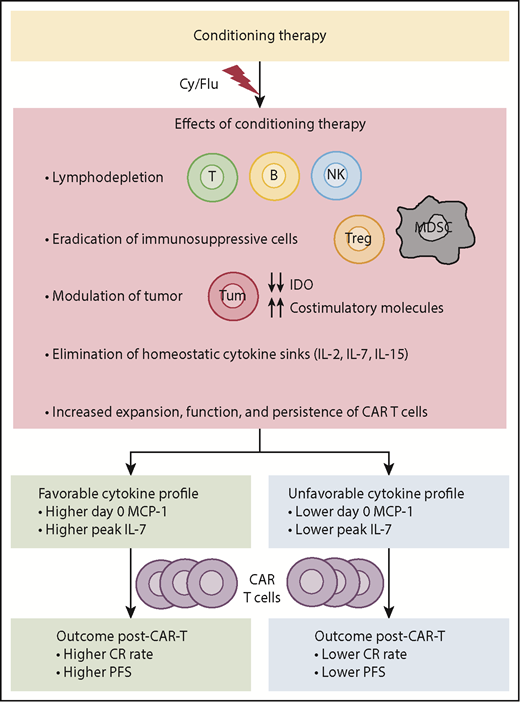
Effects of conditioning therapy and its impact on efficacy of CAR T-cell therapy. IDO, indoleamine 2,3-dioxygenase; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells.
Effects of conditioning therapy and its impact on efficacy of CAR T-cell therapy. IDO, indoleamine 2,3-dioxygenase; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells.
The approval of 2 anti-CD19 CAR T-cell therapy products, axicabtagene ciloleucel and tisagenlecleucel, represents a major advance for the management of patients with relapsed or refractory large B-cell lymphoma.2-4 Although overall response rates of >80% have been reported with these and other anti-CD19 CAR T-cell therapies,2-5 durable remissions are observed in less than half the patients, highlighting the need for better understanding of the mechanisms associated with response and resistance in order to develop novel strategies to further improve their efficacy.
Studies conducted in patients with metastatic melanoma over a decade ago demonstrated that a lymphodepleting conditioning regimen prior to adoptive cell transfer dramatically improves the efficacy of therapy with in vitro expanded tumor-infiltrating lymphocytes.6 Lymphodepleting conditioning regimen likely works by multiple mechanisms, including eliminating sinks for homeostatic cytokines, such as interleukin-2 (IL-2), IL-7, and IL-15, eradicating immunosuppressive elements, such as regulatory T cells and myeloid-derived suppressor cells, inducing costimulatory molecules and downregulating indoleamine 2,3-dioxygenase in tumor cells, and promoting expansion, function, and persistence of adoptively transferred T cells (see figure).6-8 These experiences led to the use of lymphodepleting conditioning in clinical trials with CAR T-cell therapy. Kochenderfer et al showed an association between an increase in serum IL-15 levels post-lymphodepletion and clinical response after anti-CD19 CAR T-cell therapy.9 Turtle and colleagues showed that the addition of fludarabine to cyclophosphamide (Cy/Flu) in the lymphodepleting regimen was associated with improved anti-CD19 CAR T-cell expansion and persistence and better clinical outcome compared with non-Flu lymphodepleting regimens in patients with non-Hodgkin lymphoma.10 Other studies indicated that the magnitude of the CAR T-cell expansion in vivo with respect to both the peak and the area under the curve over the first month may be associated with response and/or durability.2 However, prior reports evaluated a small number of factors and performed only univariate analysis to identify features associated with clinical outcome after CAR T-cell therapy.
Hirayama and colleagues performed comprehensive multivariable analysis of 132 factors, including clinical and treatment characteristics, serum biomarkers, and CAR T-cell manufacturing and pharmacokinetic data to identify biomarkers associated with complete response (CR) and progression-free survival (PFS) after anti-CD19 CAR T-cell therapy in patients with aggressive B-cell non-Hodgkin lymphoma who received either low- or high-intensity Cy/Flu lymphodepletion. In multivariable analysis, they observed that lower prelymphodepletion serum lactate dehydrogenase reflecting less aggressive disease and greater increase in serum monocyte chemoattract protein-1 (MCP-1) concentration in response to lymphodepletion were associated with better probability of achieving a CR and better PFS. In addition, higher serum IL-7 peak after CAR T-cell infusion was also associated with better PFS. Patients receiving high-intensity lymphodepletion had a higher probability of achieving a favorable cytokine profile, defined as day 0 MCP-1 and peak IL-7 concentrations above their respective medians, compared with those receiving low-intensity lymphodepletion. Within the subgroup of patients who received high-intensity lymphodepletion, the PFS benefit was primarily observed in those achieving a favorable cytokine profile, and multivariable modeling showed that a favorable cytokine profile but not lymphodepletion intensity was associated with better PFS. These observations provide novel insights into the mechanisms by which lymphodepleting conditioning might enhance the efficacy of adoptive T-cell therapies.
The results by Hirayama et al suggest that the biological effects of the lymphodepleting regimen are likely more important than the intensity of lymphodepletion to improve CAR T-cell efficacy. Although this study identified novel biomarkers associated with clinical outcome after CAR T-cell therapy, it also raises a number of questions. For example, it is unclear why only a subset of the patients who received high-intensity lymphodepletion achieved a favorable cytokine profile. Whether it is because of differences in the pharmacokinetics of Cy/Flu, baseline immune profiles, or tumor characteristics in these patients needs to be studied. It is unknown how higher MCP-1 and IL-7 concentrations lead to better PFS. It is possible that IL-7 acts by promoting CAR T-cell proliferation, survival, and persistence,7 but the mechanism for MCP-1 is less obvious. It is also unknown whether the increase in these markers is solely due to lymphodepletion or to other biological effects of the lymphodepletion regimen. Additional studies to elucidate these mechanisms and confirmation in larger studies are needed before considering systemic administration of IL-7 and/or MCP-1 as a strategy to improve CAR-T efficacy. As the kinetics of CAR T-cell expansion in vivo may be dependent on the costimulatory domain used (eg, CD28 vs CD137) as well as the maturation phenotype of the CAR T cells, it remains to be determined whether the biomarkers identified here will have prognostic significance with other CAR T-cell products that may have different CAR design and/or different manufacturing protocol. Studies to investigate whether the effects of an unfavorable cytokine profile can be overcome by additional engineering of the CAR T cells are also warranted. Finally, as outlined above, because accumulating evidence suggests that Cy/Flu and other regimens administered prior to adoptive T-cell therapies likely work by multiple mechanisms besides lymphodepletion, a more appropriate term for these approaches might be “conditioning regimen” rather than “lymphodepleting regimen,” and future research should explore strategies to optimize conditioning regimens to improve clinical outcomes after CAR T-cell therapy.
Conflict-of-interest disclosure: S.S.N. has received research support from Kite/Gilead, Celgene, Cellectis, Poseida, Merck, Acerta, Karus, BMS, and Unum Therapeutics and served as consultant and advisory board member for Kite/Gilead, Celgene, Novartis, Unum Therapeutics, Pfizer, Merck, Precision Biosciences, CellMedica, and Incyte.