In this issue of Blood, Muia et al1 and Zhu et al,2 using complementary approaches, provide important insights into the structure and function of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin-1 repeats, member 13), identifying critical structural features and interactions that allosterically regulate its proteolytic activity on von Willebrand factor (VWF).
Thrombotic thrombocytopenic purpura (TTP) is a devastating and life-threatening disease characterized by the extensive deposition of occlusive platelet and VWF-rich thrombi in the microcirculation. TTP is caused by a deficiency in the VWF-cleaving protease ADAMTS13, which is the only known protein to regulate the adhesive function of VWF. Increasing evidence shows that VWF is involved in many disorders, including arterial and deep vein thrombosis, stroke, atherosclerosis, sickle cell crisis, sepsis, and other thrombotic microangiopathies. There is increasing interest in using ADAMTS13 not only to treat TTP but also to control diverse VWF-related thromboses. Therefore, an improved understanding of its mechanism of action is crucial for its potential usage.
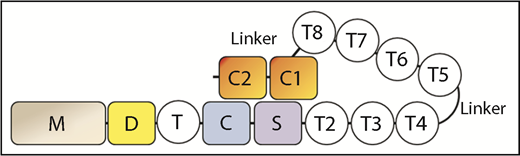
ADAMTS13 is a metalloprotease consisting of the following domains: a metalloprotease (M), disintegrin-like (D), thrombospondin-1 repeat (T), Cys-rich (C), spacer (S), 7 additional thrombospondin-1 repeats (T2-T8), and 2 CUB domains (CUB1-2) (see figure). Although the MDTCS proximal domains are sufficient to cleave a peptide substrate based on the VWF-A2 domain cleavage site sequence, the distal T2-T8 and CUB1-2 domains are required for cleaving VWF multimers under shear stress. Deletion of the distal domains impairs the ability of ADAMTS13 to cleave VWF multimers under shear, but surprisingly enhances its ability to cleave the peptide substrate.3 This finding led to the idea that the distal domains partially interfere with the proximal domains to maintain the molecule in an autoinhibited state. Consistent with this idea, several monoclonal antibodies specific for the distal domains are also able to relieve autoinhibition.4 These findings led to the hypothesis that ADAMTS13 assumes a native autoinhibited conformation in which the distal domains interact with the proximal domains, partially inhibiting access to the active center of the protease. Truncation, interaction with antibodies, or change in pH, can allosterically activate the proximal metalloprotease domains. Zanardelli et al showed in surface plasmon resonance studies that the distal domains of ADAMTS13 could bind the D4-CK domain of VWF under low shear stress or static conditions,5 suggesting that this initial interaction between ADAMTS13 and VWF not only could ctivate ADAMTS13 but also juxtapose ADAMTS13 to further interact with exosites in the A2 domain of VWF that were exposed by high shear stress.6 If the cleavage site in the VWF-A2 domain is inaccessible, ADAMTS13 would dissociate from VWF and revert to the autoinhibited state.
Crystallographic studies of the DTCS fragment of ADAMTS13 showed that the M and T2 domains that flank the DTCS fragment should be situated at opposite ends of the fragment.7 To enable CUB1-2 to contact MDTCS, a reversal in the direction of the polypeptide chain must have occurred in the distal domains. In this issue, Muia et al carried out phylogenetic analyses on ADAMTS13 and observed high variability in the number of distal T domains in the ADAMTS13 of 206 species. Pigeon ADAMTS13, which contains only 3 distal T repeats, preserves allosteric regulation. A similar deletion of the T3 to T6 domains in human ADAMTS13, retaining only 3 distal T repeats (T2, T7, and T8), also produces a minimal molecule that preserves allosteric regulation of its peptide cleavage as well as VWF multimer cleavage activities. These studies show that 3 distal T repeats are sufficient to mediate polypeptide chain reversal and preserve interactions that mediate allosteric regulation. In an accompanying paper in this issue, Zhu et al used small-angle X-ray scattering to characterize the molecular envelopes of native and truncated human ADAMTS13 molecules. These studies provided evidence of a hairpin structure in which the 29-residue flexible linker sequence between T4 and T5 apparently formed the apex, and T2-T4 and T5-T8 formed the 2 arms of the hairpin. They also provided supporting evidence for the stable interaction of MDTCS with distal T7, T8, and CUB1. Another 58-residue linker sequence between T8 and CUB1 also facilitated interaction of CUB1 with MDTCS to mediate allosteric regulation. Although not at atomic resolution, these studies provided key evidence of intramolecular interactions essential for allosteric regulation.
Information from the 2 papers provides important insights into the molecular mechanism of allosteric regulation in ADAMTS13. This mechanism includes a substrate-induced conformational change that converts ADAMTS13 into an activated state, primed to engage and cleave the substrate with high specificity. This improved understanding is crucial for the development of ADAMTS13 as a therapeutic agent to treat VWF-related thrombotic disorders.
Conflict-of-interest disclosure: D.W.C. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal