Abstract
Bone disease is a cardinal complication of multiple myeloma that affects quality of life and survival. Osteocytes have emerged as key players in the development of myeloma-related bone disease. Along with other factors, they participate in increased osteoclast activity, decreased osteoblast function, and immunosuppressed marrow microenvironment, which deregulate bone turnover and result in bone loss and skeletal-related events. Denosumab is a novel alternative to bisphosphonates against myeloma bone disease. Special considerations in this constantly evolving field are thoroughly discussed.
Introduction
Bone disease affects up to 80% of the newly diagnosed patients with multiple myeloma (NDMM).1 These patients present with substantial bone pain, and they are at high risk for developing skeletal-related events (SREs), such as pathological fractures, spinal cord compression, and need for radiotherapeutic or surgical intervention.2 Whole-body low-dose computed tomography is currently the new standard for evaluating myeloma bone disease (MBD).3,4 Regarding MBD therapeutics, there is a vivid interest in translating the complex underlying pathophysiology into effective targeted agents.5
Biology of MBD and the emerging role of osteocytes
Physiological bone remodeling is highly dependent on the fine-tuned interactions among the bone matrix, osteocytes, osteoclasts, osteoblasts, and immune cells.6 MBD is characterized by significant deregulation in all these aspects; the ultimate effect is increased osteoclast activity and suppressed osteoblast function, leading to bone loss. Osteocytes play a major role in these pathways through the secretion of receptor activator of NF-κB ligand (RANKL), sclerostin, Dickkopf-1 (DKK-1), and other factors that have not been well studied (Figure 1).7-9 MM cells lead osteocytes to apoptosis to alter the bone marrow microenvironment and provide a premetastatic niche for the MM cells.10 Indeed, MM patients have reduced number of viable osteocytes, which is associated with increased bone disease burden.11 In contrast, proteasome inhibitors, which have a positive effect on bone formation in MM, have been shown to restore osteocyte viability by reducing autophagy and apoptosis in MM patients.12
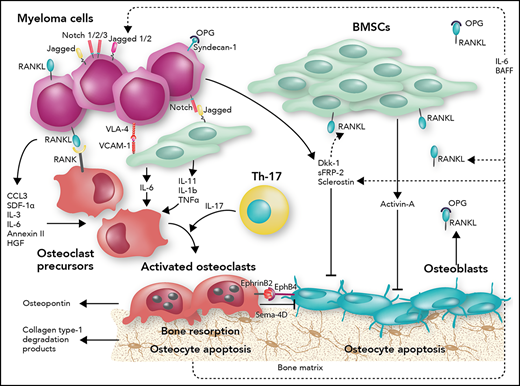
Schematic overview of MBD. The intercellular interactions between bone marrow stromal cells (BMSCs) and MM cells, along with the involvement of immune cells, such as Th17 cells, induce cytokine release (interleukin-1b [IL-1b], IL-3, IL-6, IL-11, and IL-17) and secretion of proosteoclastogenic factors such as tumor necrosis factor α (TNF-α), chemokine (C-C motif) ligand 3 (CCL3), stromal cell derived factor-1α (SDF-1α), and annexin 2 in the bone marrow microenvironment. These cytokines promote increased osteoclast activity and inhibit osteoblastogenesis. Adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) on BMSCs and very late antigen-4 (VLA-4) on MM cells mediate cell-to-cell contact. Notch, expressed by MM cells, binds to Jagged, expressed by neighboring MM cells and BMSCs, and activates intracellular cascades favoring RANKL production. RANKL, expressed by both BMSCs and MM cells, binds directly to RANK on osteoclast precursors and promotes osteoclastogenesis. Syndecan-1 on MM cells binds and inactivates osteoprotogerin (OPG), the RANKL soluble decoy receptor. Osteoclasts also produce factors sustaining MM cell growth and survival, such as osteopontin. Furthermore, osteocytes and MM cells produce soluble factors that inhibit osteoblastogenesis such as DKK-1, sFRP-2, and sclerostin. Activin-A secreted by BMSCs also impedes osteoblast production. EphB4 on osteoblasts and BMSCs binds to EphrinB2 on osteoclasts and results in bidirectional signaling that ultimately induces osteoclastogenesis and impedes osteoblastogenesis. Moreover, myeloma cells and osteoclasts produce semaphorin-4D (Sema-4D) and further inhibit the osteoblasts. Osteocyte apoptosis increases RANKL and sclerostin production to increase osteoclast activity, suppress osteoblast differentiation, and increase myeloma growth through bidirectional Notch signaling. BAFF, B cell–activating factor; HGF, hepatocyte growth factor. Professional illustration by Somersault18:24.
Schematic overview of MBD. The intercellular interactions between bone marrow stromal cells (BMSCs) and MM cells, along with the involvement of immune cells, such as Th17 cells, induce cytokine release (interleukin-1b [IL-1b], IL-3, IL-6, IL-11, and IL-17) and secretion of proosteoclastogenic factors such as tumor necrosis factor α (TNF-α), chemokine (C-C motif) ligand 3 (CCL3), stromal cell derived factor-1α (SDF-1α), and annexin 2 in the bone marrow microenvironment. These cytokines promote increased osteoclast activity and inhibit osteoblastogenesis. Adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) on BMSCs and very late antigen-4 (VLA-4) on MM cells mediate cell-to-cell contact. Notch, expressed by MM cells, binds to Jagged, expressed by neighboring MM cells and BMSCs, and activates intracellular cascades favoring RANKL production. RANKL, expressed by both BMSCs and MM cells, binds directly to RANK on osteoclast precursors and promotes osteoclastogenesis. Syndecan-1 on MM cells binds and inactivates osteoprotogerin (OPG), the RANKL soluble decoy receptor. Osteoclasts also produce factors sustaining MM cell growth and survival, such as osteopontin. Furthermore, osteocytes and MM cells produce soluble factors that inhibit osteoblastogenesis such as DKK-1, sFRP-2, and sclerostin. Activin-A secreted by BMSCs also impedes osteoblast production. EphB4 on osteoblasts and BMSCs binds to EphrinB2 on osteoclasts and results in bidirectional signaling that ultimately induces osteoclastogenesis and impedes osteoblastogenesis. Moreover, myeloma cells and osteoclasts produce semaphorin-4D (Sema-4D) and further inhibit the osteoblasts. Osteocyte apoptosis increases RANKL and sclerostin production to increase osteoclast activity, suppress osteoblast differentiation, and increase myeloma growth through bidirectional Notch signaling. BAFF, B cell–activating factor; HGF, hepatocyte growth factor. Professional illustration by Somersault18:24.
Increased osteoclast activity
A cardinal signaling cascade regulating osteoclast maturation and activation is the RANK/RANKL pathway. RANKL is produced mainly by osteocytes, but also by activated lymphocytes, BMSCs, and endothelial cells, and promotes osteoclast activity by binding to RANK on the membrane of osteoclastic lineage cells. OPG is secreted by osteoblasts, BMSCs, and osteocytes and antagonizes the interaction between RANKL and RANK.13,14 MM cells degrade OPG through the membrane syndecan-1 system.15 Therefore, the increased RANKL/OPG ratio favors bone destruction, and importantly, it affects prognosis of NDMM patients.16
Another aberrant pathway inducing osteoclast activity is the Notch signaling pathway. Both members of the Notch family and their Jagged ligands are expressed in the membranes of MM cells, enabling homotypical and heterotypical interactions with the same or adjacent cells, respectively. The subsequently activated intracellular cascade ultimately results in the increase of RANKL production by MM cells. However, in the myeloma microenvironment, BMSCs and osteocytes remain the main sources of RANKL.17,18 Other factors favoring osteoclastogenesis and osteoclast-mediated bone loss include chemokines such as CCL3, SDF-1α, osteopontin, interleukins, annexin 2, members of the transforming growth factor β (TGFβ) superfamily such as activin-A, and members of the TNF superfamily such as TNF-α and BAFF (Figure 1).19-26
Suppressed osteoblast activity
The Wingless-type (Wnt) and β-catenin pathway is a principal regulator of osteoblast differentiation and bone homeostasis.27 Osteocytes and MM cells express Wnt antagonists such as sclerostin, DKK-1, and soluble frizzled-related proteins and suppress osteoblast activity.28,29 Sclerostin is an osteocyte product associated with abnormal bone remodeling; it impedes the activation of the canonical Wnt pathway and inhibits osteoblast maturation, impairs bone mineralization, and induces osteoblast apoptosis through caspase cascade activation.30,31 Sclerostin inhibition with monoclonal antibodies in preclinical MM models restores the deregulated bone metabolism and decreases bone fragility.29,32 DKK-1 has a synergistic effect with sclerostin and disrupts autocrine Wnt signaling and, subsequently, suppresses osteoblast differentiation and activity.28,33 Other regulatory factors of the Wnt pathway that are found deregulated in MM include periostin, runt-related transcription factor 2, and growth factor independence-1.34-36 Furthermore, members of the TGFβ and TNF superfamilies along with interleukins are also implicated in favoring osteoblast suppression, whereas osteoclasts and myeloma cells further inhibit osteoblasts through semaphorin-4D (Figure 1).37-39 Interestingly, proteasome inhibitors promote osteoblast differentiation by upregulating the intracellular β-catenin pathway independently of Wnt signaling; thus, they have an anabolic effect on myelomatous bone.40-42
Bone marrow microenvironment
In the bone marrow microenvironment, there is a constant crosstalk among the different cell subtypes.43 Homing of MM cells is favored by their adhesion to BMSCs through the VLA-4/VCAM-1 integrin system.44 Notch bidirectional signaling also mediates interactions among MM cells, BMSCs, and osteocytes and leads to significant alterations in the bone marrow microenvironment, promoting MM proliferation and bone destruction.17,18 The dysregulated EphrinB2/EphB4 signaling in MM also impairs the normal interaction between osteoclasts and osteoblasts, which finally results in increased bone loss.45 Osteoclasts potentiate the immunosuppressive microenvironment by promoting the expansion of Th17 lymphocytes and myeloid-derived suppressor cells, whereas they inhibit the cytotoxic T and NK cells against MM cells.46,47 The upregulation of immune checkpoint molecules and T-cell metabolism regulators provides the rationale for targeted monoclonal antibodies against programmed death ligand 1 and CD38.47,48 Interestingly, the interplay between MM cells and mature osteoblasts may provide a unique niche for MM cells to be maintained in quiescence, whereas osteoblast dysfunction or osteoclast remodeling of the endosteal niche allows their reactivation.49-51
Treatment of MBD
Bisphosphonates
Bisphosphonates remain the cornerstone for the management of MBD (Table 1).52-58 Bisphosphonates are pyrophosphate analogs that bind to exposed bone areas of hydroxyapatite crystals. During bone remodeling, they are absorbed by the osteoclasts, and they inhibit intracellular farnesyl pyrophosphate synthase; thus, bisphosphonates impair osteoclastogenesis and disrupt osteoclast activity.59
Summary of major randomized clinical trials on bone-targeting agents for treatment of MBD
| Study . | Treatment drug . | Treatment schedule . | Patients, n . | Median time to first SRE, mo . | SRE incidence, % . | ONJ incidence, % . | Renal toxicity, % . |
|---|---|---|---|---|---|---|---|
| 52 | Pamidronate vs placebo | 90 mg of pamidronate every 4 wk for 9 cycles | 196 vs 181 | Shorter in placebo group (P = .001) | 24 vs 41 (P < .001) | NR | NR |
| 53 | ZA vs pamidronate | 4 or 8 mg of ZA IV or 90 mg of IV pamidronate every 3-4 wk for 12 mo | 129 vs 65 | 12.5 vs 9.4 | NR | NR | NR |
| 54 | Pamidronate | 30 vs 90 mg of pamidronate | 252 vs 252 | 10.2 vs 9.2 (P = .63) | 33.7 vs 35.2 | 0.8 vs 3.2 | NR |
| 55,56 | ZA vs clodronate | 4 mg of ZA IV every 3-4 wk or 1600 mg of clodronic acid orally daily | 981 vs 979 | NR | 27 vs 35 (P = .0004) | 4 vs <1 | Similar for 2 treatment groups (P = .55) |
| 57 | ZA | ZA every 12 vs every 4 wk | 139 vs 139 | NR | 55 vs 60 | NR | NR |
| 58 | Denosumab vs ZA | 120 mg of denosumab sc plus placebo IV or ZA 4 mg IV plus placebo sc every 4 wk | 859 vs 859 | 22.8 vs 24 (Pnoninferiority = .01) | 43.8 vs 44.6 | 4.1 vs 2.8 | 10 vs 17.1 |
| Study . | Treatment drug . | Treatment schedule . | Patients, n . | Median time to first SRE, mo . | SRE incidence, % . | ONJ incidence, % . | Renal toxicity, % . |
|---|---|---|---|---|---|---|---|
| 52 | Pamidronate vs placebo | 90 mg of pamidronate every 4 wk for 9 cycles | 196 vs 181 | Shorter in placebo group (P = .001) | 24 vs 41 (P < .001) | NR | NR |
| 53 | ZA vs pamidronate | 4 or 8 mg of ZA IV or 90 mg of IV pamidronate every 3-4 wk for 12 mo | 129 vs 65 | 12.5 vs 9.4 | NR | NR | NR |
| 54 | Pamidronate | 30 vs 90 mg of pamidronate | 252 vs 252 | 10.2 vs 9.2 (P = .63) | 33.7 vs 35.2 | 0.8 vs 3.2 | NR |
| 55,56 | ZA vs clodronate | 4 mg of ZA IV every 3-4 wk or 1600 mg of clodronic acid orally daily | 981 vs 979 | NR | 27 vs 35 (P = .0004) | 4 vs <1 | Similar for 2 treatment groups (P = .55) |
| 57 | ZA | ZA every 12 vs every 4 wk | 139 vs 139 | NR | 55 vs 60 | NR | NR |
| 58 | Denosumab vs ZA | 120 mg of denosumab sc plus placebo IV or ZA 4 mg IV plus placebo sc every 4 wk | 859 vs 859 | 22.8 vs 24 (Pnoninferiority = .01) | 43.8 vs 44.6 | 4.1 vs 2.8 | 10 vs 17.1 |
NR, not reported; ONJ, osteonecrosis of the jaw; sc, subcutaneously; ZA, zoledronic acid.
Which bisphosphonate to choose?
Elucidation of the field was attempted in a recently updated metaanalysis including 7293 patients.60 Although all agents showed substantial efficacy in reducing SREs and bone pain, none of the approved bisphosphonates (clodronate, pamidronate, or ZA) showed superiority over another in any of the outcomes.60 Interestingly, ZA provided a survival advantage. The Myeloma IX trial showed that ZA increased median overall survival as compared with clodronate.55 ZA may exert direct antimyeloma effects by inhibition of protein prenylation or indirect effects through downregulation of BMSC-related adhesion molecules and blocking of osteoclast activation.59,61,62
When and how to start and stop bisphosphonates?
According to the International Myeloma Working Group, all NDMM patients starting antimyeloma regimens should be evaluated for treatment with bisphosphonates, although the evidence is more robust regarding those presenting with evident MBD on imaging studies.63 Overall, bisphosphonates should be administered up to 2 years if they are well tolerated.64 On the basis of the updated results of the Myeloma IX trial, ZA should be administered until disease progression in patients not achieving very good partial response or better.56 The continuation of ZA in patients with inactive MM and other bisphosphonates beyond the 2 years is at the physician’s discretion on an individualized basis.63,64 Bisphosphonates should be reinitiated at the time of relapse, if they have been previously interrupted.63
The standard dosing schedule for patients without renal impairment for ZA is 4 mg every 3 to 4 weeks in a 15-minute infusion; for pamidronate, it is 90 mg every 3 to 4 weeks in a 2-hour infusion.64 In an effort to reduce toxicities, two important studies have assessed the deintensification of bisphosphonate treatment. The Z-MARK study implemented a dynamic, risk-adapted infusion of ZA monthly or every 3 months based on the levels of the urinary N-telopeptide of type 1 collagen, development of SREs, and disease progression. This strategy provided low SRE rates of 5.8% and 4.9% during the first and second years on study, respectively, and had a favorable safety profile.65 Another phase 3 trial showed that monthly administration of pamidronate at 30 mg was not inferior to 90-mg dosing in terms of physical function assessment and median time to first SRE, while producing fewer cases of ONJ.54
Are there any alternatives to bisphosphonates?
Denosumab
Denosumab is a fully human, monoclonal antibody of the immunoglobulin G2 class that binds to RANKL with high specificity and prevents its interaction with RANK; thus, it mimics the effect of endogenous OPG and inhibits bone resorption.66 Until early 2018, denosumab was licensed for postmenopausal women with osteoporosis and patients with either primary giant cell tumors of the bone or bone metastases secondary to solid tumors. In 2018, both the US Food and Drug Administration and European Medicines Agency granted supplemental approval for denosumab for the prevention of SREs among MM patients based on the results of the 20090482 phase 3 study.58
Denosumab vs ZA
The 20090482 phase 3 study was an international, double-blind, double-dummy, 1:1 randomized controlled trial that evaluated the efficacy and safety of denosumab compared with ZA among 1718 NDMM patients.58 After a median time on study of 17.3 and 17.6 months for the denosumab and ZA groups, respectively, denosumab was noninferior to ZA in delaying time to first SRE, which was the primary study end point. Interestingly, a post hoc landmark analysis at 15 months suggested denosumab superiority for time to first SRE. Although the data were rather immature to reach firm conclusions on the secondary end point of overall survival, denosumab demonstrated a clinically meaningful median progression-free survival (PFS) benefit of 10.7 months (P = .036) compared with ZA on top of standard-of-care first-line antimyeloma treatment.58 A supplemental analysis according to prespecified patient subgroups on the latter exploratory end point was recently provided. It seems that the PFS impact of denosumab is more pronounced among NDMM patients receiving first-line therapy based on proteasome inhibitors without immunomodulatory drugs (P = .019) and those who underwent autologous stem cell transplantation (P = .002).67
When and how to start and stop denosumab?
Denosumab is administered subcutaneously at 120 mg every 4 weeks in NDMM patients.58 Currently, there is an open debate on whether and under what circumstances denosumab treatment could be discontinued. Because of a lack of pertinent data on solid malignancies and MM, there are recommendations based on osteoporosis studies. In contrast to bisphosphonates, denosumab is not incorporated into the bone matrix and does not show prolonged effects after its discontinuation.68 Denosumab cessation leads to reduced bone mineral density and increased risk of fractures after 12 to 18 months after its discontinuation. This rebound effect may be attributed to the resultant high soluble RANKL/OPG ratio, which is associated with massive maturation of previously dormant osteoclast precursors.68 Therefore, immediate bridging with antiresorptive agents (ie, 1 dose of IV bisphosphonate) is considered vital among patients who discontinue denosumab to preserve the attained favorable effect on bone indices in postmenopausal osteoporosis patients.69 In MM, there are no data on how to stop denosumab, and the drug has been licensed for continuous use. Studies on this topic are urgently needed to provide specific recommendations. Alternatively, especially among patients at high risk for SREs, denosumab could be continuously administered, taking into consideration the results of the 10-year follow-up of the FREEDOM and open-label extension studies demonstrating a manageable toxicity profile, continuous improvement in BMD, and decreasing fracture risk among postmenopausal women with osteoporosis.70
Evaluation of the safety profile
Renal toxicity is a major concern among MM patients receiving bispshosphonates. ZA causes acute tubular necrosis, whereas pamidronate impairs glomerular function. Therefore, close monitoring of creatinine clearance and urine protein, along with the optimal dose modifications, is essential.64 In the 20090482 trial, renal impairment was more frequent in the ZA (17%) compared with the denosumab group (10%).58 Denosumab may be a viable option for patients with renal insufficiency (creatinine clearance, 30-60 mL per minute), but it has to be further investigated in clinical trials for patients with creatinine clearance <30 mL per minute (NCT02833610).
Conclusion
Although bisphosphonates have been the mainstay of MBD for several years, denosumab may be an acceptable, cost-effective alternative, considering its noninferiority in terms of SRE prevention and the potential to minimize renal toxicity and improve PFS.71 Further understanding of MBD biology may be translated into novel combinations of antimyeloma and bone-targeting agents that would both reduce disease burden and restore bone metabolism. Antisclerostin agents seem to be the more promising to enter into clinical development for MM to date.
Authorship
Contribution: E.T., I.N.-S., and M.A.D. designed, wrote, and critically revised the manuscript.
Conflict-of-interest disclosure: E.T. has received honoraria from Amgen, Celgene, Genesis, Janssen, Novartis, Bristol-Myers Squibb, and Takeda; is a member of the steering committee for Amgen and Takeda and a member of the independent data monitoring committee for Celgene; and has received research grants from Amgen, Janssen, and Takeda. M.A.D. has received honoraria from Amgen, Celgene, Genesis, Janssen, Bristol-Myers Squibb, and Takeda. I.N.-S. declares no competing financial interests.
Correspondence: Evangelos Terpos, Department of Clinical Therapeutics, Medical School, National and Kapodistrian University of Athens, Alexandra General Hospital, 80 Vas. Sofias Ave, 11528 Athens, Greece; e-mail: eterpos@med.uoa.gr and eterpos@hotmail.com.

![Figure 1. Schematic overview of MBD. The intercellular interactions between bone marrow stromal cells (BMSCs) and MM cells, along with the involvement of immune cells, such as Th17 cells, induce cytokine release (interleukin-1b [IL-1b], IL-3, IL-6, IL-11, and IL-17) and secretion of proosteoclastogenic factors such as tumor necrosis factor α (TNF-α), chemokine (C-C motif) ligand 3 (CCL3), stromal cell derived factor-1α (SDF-1α), and annexin 2 in the bone marrow microenvironment. These cytokines promote increased osteoclast activity and inhibit osteoblastogenesis. Adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) on BMSCs and very late antigen-4 (VLA-4) on MM cells mediate cell-to-cell contact. Notch, expressed by MM cells, binds to Jagged, expressed by neighboring MM cells and BMSCs, and activates intracellular cascades favoring RANKL production. RANKL, expressed by both BMSCs and MM cells, binds directly to RANK on osteoclast precursors and promotes osteoclastogenesis. Syndecan-1 on MM cells binds and inactivates osteoprotogerin (OPG), the RANKL soluble decoy receptor. Osteoclasts also produce factors sustaining MM cell growth and survival, such as osteopontin. Furthermore, osteocytes and MM cells produce soluble factors that inhibit osteoblastogenesis such as DKK-1, sFRP-2, and sclerostin. Activin-A secreted by BMSCs also impedes osteoblast production. EphB4 on osteoblasts and BMSCs binds to EphrinB2 on osteoclasts and results in bidirectional signaling that ultimately induces osteoclastogenesis and impedes osteoblastogenesis. Moreover, myeloma cells and osteoclasts produce semaphorin-4D (Sema-4D) and further inhibit the osteoblasts. Osteocyte apoptosis increases RANKL and sclerostin production to increase osteoclast activity, suppress osteoblast differentiation, and increase myeloma growth through bidirectional Notch signaling. BAFF, B cell–activating factor; HGF, hepatocyte growth factor. Professional illustration by Somersault18:24.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/14/10.1182_blood-2018-11-852459/4/m_blood852459f1.png?Expires=1769088116&Signature=3xLJsOswPVpnxtYEQPrzBV~0tiMfEdoRzZJlkbd7YcOIUDrWvrlbkb3mf3Tk5jnougjL32pRiczmflWfJpjdLlsBY7F18nuW8yYWta8f-n-jocc3XHbmSn-itthXkMqgVarR~8ghBvQUug7IulOkUcR2TzQv~Rv1AxXqUXFlM8PVlvRX8BZJkzlUombh5oy-ya04VRjLhR6~rmw2qxC6DZ-GYaBbW~WLxfNx1cNXqNKWs5V6F-lWhfJM60A3NFt1fdGgiszc8EQydqqPlYmeGo7k9mWSK4O8S8x4df7jqOid5n8pwlOwBEZrSpwMoVNlPGpwlfYwpjS7GdsbCxMjEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)