Abstract
Follicular lymphoma (FL) is the most frequently occurring indolent non-Hodgkin lymphoma, with generally favorable outcomes but a variable clinical course. Recent studies have elucidated the consistent and reproducible frequency of early disease progression in FL, occurring in ∼20% of patients. Relapse of FL within 24 months of chemoimmunotherapy (POD24) is now established as a robust marker of poor survival, leading to increased risk of death. Currently, there is no established method of identifying patients at risk for early disease progression at the time of their FL diagnosis. However, numerous studies worldwide are investigating clinical, pathologic, and radiographic biomarkers to help predict POD24, thereby improving subsequent outcomes and adapting therapy based on individual risk. There is also a paucity of standardized treatments for patients with POD24, but investigations are ongoing testing novel targeted therapies and autologous stem cell transplantation strategies. This review provides an overview of early-relapsing FL and our approach to patient management based on recent available data.
Introduction
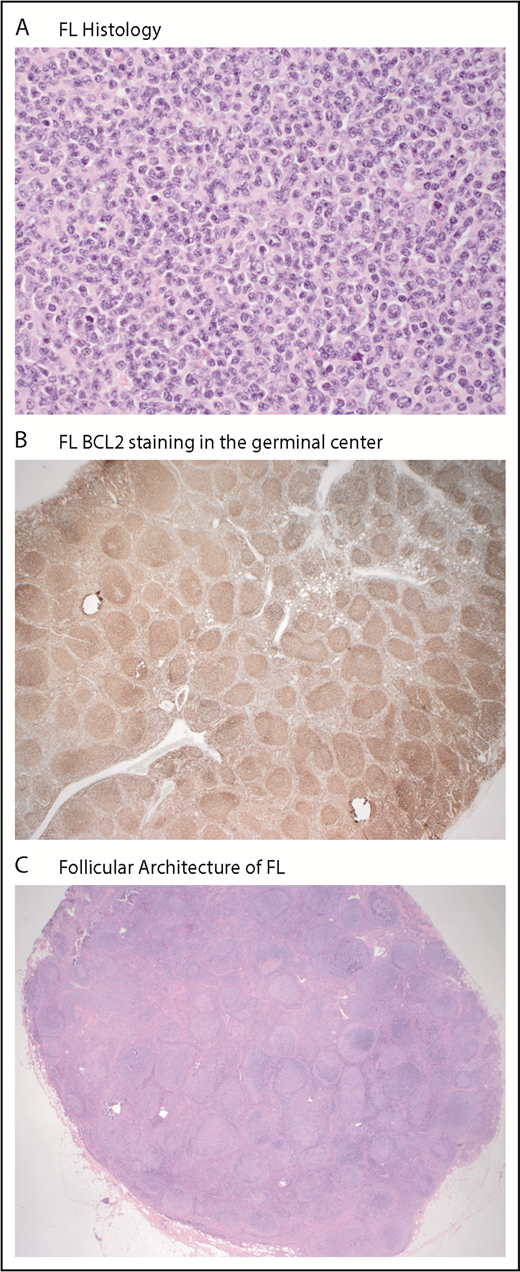
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL), representing 20% to 30% of all cases.1,2 FL is derived from the germinal center and characterized by well-preserved follicles, where malignant cells typically coexpress CD10 (89% of cases), CD20, and B-cell leukemia/lymphoma 2 protein (BCL2; 85% of cases; Figure 1).3,4 FL tumors arise from a common progenitor cell composed of numerous subclones, with 1 dominant clone.5,6 The genetic event characteristic of 90% of FL cases is a translocation involving the antiapoptotic BCL2 gene (18q21.3) on chromosome 18 to the transcriptional enhancer of the immunoglobulin heavy chain gene locus on chromosome 14 (q32;q21), resulting in a survival advantage from constitutive overexpression of BCL2.7 This cooccurs with other genetic alterations, leading to dysregulation of epigenetic modifiers, clonal expansion, and additional genomic modifications leading to FL development.
Typical immunohistochemistry of follicular lymphoma. (A) Predominance of small centrocytes in follicular lymphoma (hematoxylin and eosin; original magnification ×400). (B) BCL2 expression in follicular lymphoma (immunohistochemical stain for BCL2; original magnification ×40). (C) Nodal architecture is effaced by follicular lymphoma (hematoxylin and eosin; original magnification ×12.5). Images supplied by Richard Burack, Wilmot Cancer Institute.
Typical immunohistochemistry of follicular lymphoma. (A) Predominance of small centrocytes in follicular lymphoma (hematoxylin and eosin; original magnification ×400). (B) BCL2 expression in follicular lymphoma (immunohistochemical stain for BCL2; original magnification ×40). (C) Nodal architecture is effaced by follicular lymphoma (hematoxylin and eosin; original magnification ×12.5). Images supplied by Richard Burack, Wilmot Cancer Institute.
Although considered incurable, the development of anthracycline-containing chemotherapy and use of anti-CD20 monoclonal antibody rituximab have improved outcomes for FL with each passing decade. Currently, median survival approaches nearly 20 years.8 However, most patients have a relapsing and remitting pattern of illness, requiring numerous treatments in the course of their lifetime. Early recurrence of FL after frontline chemoimmunotherapy occurs consistently in 20% of patients and has emerged as one of the best available predictors of poor survival.9 There are challenging questions in approaching patients with early-relapsing FL. (1) What is the precise definition of early disease recurrence, and what is the magnitude of impact on survival? (2) What is the current understanding of clinical and biologic factors predicting early relapse at the time of diagnosis? (3) What surveillance or diagnostic approaches should we use to identify high-risk FL? (4) What therapeutic approaches should be used for early relapse? This review will provide an overview of the prognostic impact of early relapse and our current approach to identifying and treating these patients, while incorporating recent developments in our understanding of the clinical and biologic features of this population.
How do we define early relapse of FL?
Early relapse has been defined broadly as FL recurrence within 2 years of chemoimmunotherapy or within 2 years of diagnosis. For the purposes of this review, we will define early relapse as progression of disease within 24 months of frontline FL chemoimmunotherapy initiation (POD24). This definition is based upon disease progression assessed clinically and/or radiographically. However, histologic confirmation of disease progression is recommended to aid in subsequent management. The initial selection of 24 months was selected as the time during which most early events occurred consistently and reproducibly across FL studies.10-12 Moreover, the observation that ∼20% of patients experience disease progression by 24 months has been validated multiple times by independent investigators.13-17
The impact of early FL progression on patient survival
The magnitude of the impact of early recurrence on FL survival was previously unclear. In a pivotal study, we evaluated the prognostic significance of early FL progression using the prospective, observational National LymphoCare Study (NLCS).9 We analyzed 2727 patients with FL enrolled between 2004 and 2007 at >200 community and academic sites in the Unites States and identified 588 patients treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Patients were excluded if they had disease progression before treatment, transformation to an aggressive histology, or observation before therapy. Patients were divided into 2 groups; those with progression of disease or death within 2 years (24 months) of diagnosis treated with R-CHOP were defined as the early progressor group, and those without progression or death within 2 years of R-CHOP were defined as the reference group. Median time to treatment with chemotherapy in this cohort was 6 weeks. Progression of disease within 2 years of R-CHOP (POD24) occurred in ∼20% of patients and was significantly associated with inferior overall survival (OS), with 50% OS at 5 years, compared with 90% in the reference group without early recurrence. Even after controlling for FL International Prognostic Index (FLIPI) score, early relapse conferred an increased risk of death, with a hazard ratio (HR) of 6.44 (95% confidence interval [CI], 4.33-9.58). Analyses performed in patients treated with rituximab and cyclophosphamide, vincristine, and prednisone (R-CVP) and rituximab with fludarabine had consistent findings. These results were validated in an independent patient cohort from the University of Iowa and the Mayo Clinic Molecular Epidemiology of Resources, where early progressors similarly had poor outcomes (34% at 5 years; HR, 19.8).
Validation studies of POD24 influence on poor survival
Several groups have independently validated these findings. The German Low Grade Lymphoma Study Group evaluated the prognostic impact of POD24 in patients treated in upfront trials using R-CHOP and a second population-based cohort treated with R-CVP from the British Columbia Cancer Agency.14 POD24 occurred in 17% to 23% of patients, with 5-year OS rates of 41% in patients from the German Low Grade Lymphoma Study Group and 26% in patients from the British Columbia Cancer Agency, compared with 91% and 86% for patients without POD24, respectively (P < .001).
The FL Analysis of Surrogacy Hypothesis investigators recently reported the largest validation of the poor impact of POD24 in a pooled data set of 5453 patients with FL treated in 13 randomized controlled upfront international clinical trials. A multivariate logistic regression model indicated that male sex, poor performance status (PS), high FLIPI risk score, and elevated baseline β2 microglobulin (B2M) were associated with increased risk of progression or death before 24 months. However, POD24 emerged as the most robust independent risk factor for poor survival, even after adjusting for those variables (HR, 5.67; P < .001).15
Maurer et al17 evaluated the impact of earlier disease-related events (within 1 year of diagnosis) in patients relapsing after chemoimmunotherapy, observation, or single-agent rituximab treated at the University of Iowa and the Mayo Clinic Molecular Epidemiology of Resources. A separate replication cohort of 412 patients from registries in Lyon, France, was also analyzed. All patients with early progression had inferior outcomes, but these were more pronounced if occurring after chemoimmunotheray (compared with single-agent rituximab or observation). Those with relapse within 12 months after chemoimmunotherapy had a standard mortality ratio of 17.63 and 19.10 (replication cohort). However, patients remaining event free at 12 months had excellent outcomes, with survival approaching that of the general population.
Type of induction chemotherapy may influence survival of patients experiencing POD24. In the GALLIUM study using obinutuzumab-based induction for frontline FL treatment, an exploratory analysis evaluated the frequency and impact of POD24. Risk of mortality from POD24 was increased, with HR of 26 (95% CI, 16.2-40.3), and risk was proportional to how early progression occurred. Obinutuzumab-based chemotherapy was associated with a 34% reduction in the number of POD24 events. However, postprogression survival was similar in all treatment groups.13 Other recent analyses of POD24 after bendamustine-based induction also suggested a decreased risk of POD24 events (9% to 12%), with similarly poor outcomes.18,19
These findings suggest that early disease-related events after chemomimmunotherapy occur regularly and reproducibly in FL. This work has generated the hypothesis that patients with POD24 are biologically distinct, possessing tumor- and/or host-related factors contributing to chemotherapy resistance, and require novel therapeutic approaches to improve poor outcomes.
Case 1
Presentation
A 48-year-old man presents for a second opinion after treatment of grade 3a FL. He had stage 3 disease at diagnosis with a bulky (12 cm) retroperitoneal mass. He received 6 cycles of R-CHOP, with complete response (CR) by positron emission tomography (PET) and marked reduction in the size of the lymph node mass. He tolerated therapy well and has been on rituximab maintenance for 1 year. Routine surveillance computed tomography (CT) scans showed an increase in a large retroperitoneal lymph node mass, new pulmonary nodules, and bony lesions. A PET scan revealed mild fluorodeoxyglucose avidity of the involved lesions (standardized uptake value, 4-7). He had a laparoscopic biopsy of the retroperitoneal mass, showing a mix of grade 2 to 3a FL. A CT-guided biopsy of a pulmonary nodule showed grade 2 FL. He has no other medical history, and PS is 0.
What clinical factors at diagnosis help identify patients who will experience future POD24?
There is currently no standardized method to prospectively identify which patients with new FL will have future POD24. Studies are ongoing to determine clinical and genetic factors predicting who is most vulnerable for early relapse. The FLIPI has been evaluated as a classifier to identify POD24 patients and has a specificity (true negative rate) of ∼60% (Table 1). Although three-quarters of patients with POD24 have a high-risk pretreatment FLIPI score, a high-risk FLIPI score at the time of FL diagnosis can also be found in ∼40% of patients without subsequent POD24. To improve upon this, other predictive models adding additional variables to the FLIPI are under study. The FL Analysis of Surrogacy Hypothesis involving 5453 patients with FL found that in addition to high-risk FLIPI score, male sex, elevated B2M, and poor PS were associated with early disease progression or death.15 In another simplified prognostic model including only increased B2M and bone marrow involvement (Primary Rituximab and Maintenance Study [PRIMA] Prognostic Index), 38% of high-risk patients subsequently experienced early disease-related events. Molecular markers are also being combined with clinical factors to improve the accuracy of identifying future POD24.
Sensitivity and specificity of prognostic indices in POD24
| . | High risk FLIPI, % . | High risk m7-FLIPI, % . | High risk POD24-PI, % . |
|---|---|---|---|
| Sensitivity | 70-78 | 43-61 | 61-78 |
| Specificity | 56-58 | 79-86 | 67-73 |
| . | High risk FLIPI, % . | High risk m7-FLIPI, % . | High risk POD24-PI, % . |
|---|---|---|---|
| Sensitivity | 70-78 | 43-61 | 61-78 |
| Specificity | 56-58 | 79-86 | 67-73 |
m7-FLIPI, new model of FLIPI incorporating mutational status of 7 frequently recurring genes; PI, prognostic index.
What biologic factors affect development of POD24?
Insights into the clonal evolution of FL have revealed that relapsed disease exhibits a complex evolutionary pattern. Recurrent FL emerges through the linear expansion of a dominant (resistant) subclone, with acquired mutations in several genes, including epigenetic modifiers. Patients with POD24 are enriched for mutations in KMT2C, TP53, BTG1, MK167, XBP1, SOCS1, IKZF3, B2M, FAS, and MYD88.20 In a study by the Leukemia and Lymphoma Molecular Profiling Project, heterozygous TP53 mutations were identified in 6% of FL cases and were associated with short remission duration and decreased progression-free survival (PFS) compared with wild type.21
The mutational status of 7 frequently recurring genes in FL has been incorporated into a clinicopathologic model to predict outcome in newly diagnosed FL. Sequencing studies performed by Pastore et al22 found mutations in epigenetic modifiers, transcriptional regulators, and nucleosome remodeling genes. When combined with PS and FLIPI score, the new model (m7-FLIPI) predicted failure-free survival.22 The m7-FLIPI accurately identified between 43% and 61% of patients with subsequent POD24 (Table 1). In a slight variation of the m7-FLIPI using 3 rather than 7 genes (termed POD24-PI), detection of future POD24 increased slightly (61% to 78%), but at the expense of lower accuracy, because nearly one-third of patients without POD24 were assigned to the high-risk group (Table 1).
The Lymphoma Study Association analyzed data from the PRIMA study using gene expression profiling (GEP) before treatment to establish which gene signatures were enriched for treatment failure and poor PFS.5 A 23-gene predictor characterized by the presence of B-cell centroblasts was implicated in greater disease aggressiveness and was capable of distinguishing patient outcome independently of the FLIPI, with validation in 3 international cohorts. This GEP discriminates patients at greater risk of POD24 (38%) compared with other prognostic models. In addition to these biomarkers, PET is under consideration for use alone and in combination with clinical and molecular markers (eg, circulating tumor DNA) to identify the highest-risk patients, but further validation is required.23,24
What surveillance/diagnostic approaches should be used to identify the high-risk patient?
In the original NLCS, relapsed FL was identified at the discretion of the treating physician (clinically, radiographically or by other means), and patients known to have transformed disease were excluded. Studies validating POD24 using population-based registries and clinical trials similarly identified patients clinically or by using prespecified surveillance strategies per protocol.13,14,16 In current practice, guidelines suggest judicious use of routine imaging for indolent lymphomas at the discretion of the treating physician,25 and recently, a retrospective analysis from Emory University reported that routine surveillance imaging does not have an impact on OS in FL.26
Currently, there are insufficient data to use the FLIPI, the m7-FLIPI, or other prognostic systems to predict for early progression. As such, outside of a clinical trial, we recommend using the FLIPI at the time of diagnosis for standard risk stratification and clinical monitoring of all FL patients after completion of initial chemoimmunotherapy.
Case 1 approach: do you recommend a biopsy?
We recommend that patients with recurrent FL undergo an excisional biopsy (Figure 2) before initiating next therapy to rule out transformation to another histology or malignancy. The precise rate of histologic transformation in POD24 is not exactly known. An analysis of the PRIMA study found that among 463 relapsing patients with FL, only 42% (194 patients) were biopsied at the time of first disease recurrence.27 Of these, 40 patients (20%) had histologic transformation. Of all the biopsies performed during the first year of follow-up, 36% were transformed. However, reasons for biopsy were not known, and data on biopsies performed during the second year of follow-up are not available, suggesting potential bias for performing biopsy in patients who were sicker or had more aggressive disease behavior. The European ARISTOTLE study also suggested biopsies upon first progression to document histologic transformation in FL occur infrequently.28 Recently presented retrospective data suggest that after bendamustine-based therapy, rates of POD24 may be lower (9% to 12%), and among those cases, transformation rates were reported in up to three-quarters of patients.18,19 However, biopsy to document histologic transformation occurred only in small numbers of patients. As such, these data require validation in larger prospective cohorts.
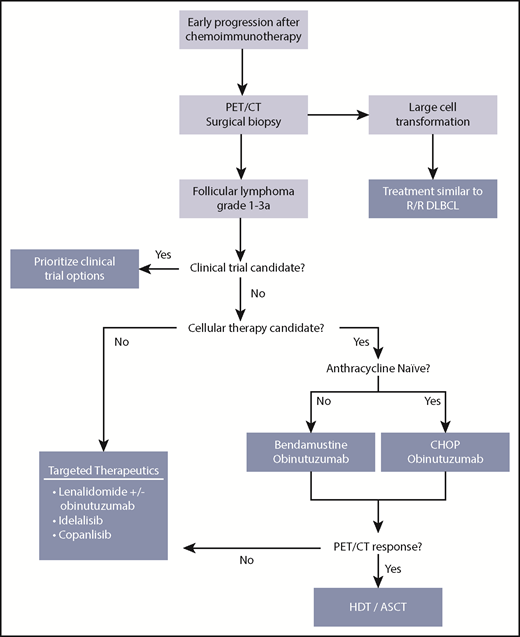
Our approach to treating patients with FL and early relapse. ASCT, autologous stem cell transplantation; DLBCL, diffuse large B-cell lymphoma; HDT, high-dose therapy; R/R, relapsed/refractory.
Our approach to treating patients with FL and early relapse. ASCT, autologous stem cell transplantation; DLBCL, diffuse large B-cell lymphoma; HDT, high-dose therapy; R/R, relapsed/refractory.
How do you treat early-relapsing FL?
Identification of novel therapies for this vulnerable group has emerged as the highest priority in lymphoma clinical trial research by the National Cancer Institute.30 Complicating matters is a lack of robust end points to know when we have achieved success for patients with early relapse. Additionally, the best approach for asymptomatic early-relapsing patients with low tumor burden has not been specifically evaluated. Response rates and duration of response are metrics being tested in clinical studies, but they have not been established as gold standards of meaningful outcome. However, the goal for any next therapy involves overcoming chemotherapy resistance, targeting mechanisms of disease, and achieving durable disease control. We consider clinical trial participation as the primary treatment option (Figure 2) for patients experiencing POD24, given the limited prospective evidence guiding treatment decisions for this population.
Is the patient a candidate for a clinical trial?
As of this writing, there are 2 prospective studies directed at the POD24 population. These include S1608 (randomized phase 2 study of obinutuzumab with CHOP or bendamusine vs umbralisib vs lenalidomide [NCT03269669]) and ZUMA 5 (a phase 2 multicenter study of axicabtagene ciloleucel in those with relapsed/refractory indolent NHL [NCT03105336]). The FRESCO study was investigating duvelisib with rituximab vs R-CHOP alone; however, it was stopped.
What is the role of standard chemoimmunotherapy?
The initial goal in patients with early relapse is to provide disease control, ideally inducing complete remission, given the association with improved outcomes after chemoimmunotherapy and consolidation stem cell transplantation.31 With a demonstrated favorable adverse effect profile and suggestion of superior disease control, bendamustine-based therapy has become a commonly used induction strategy for FL patients with symptomatic advanced-stage disease. Compared with CHOP, a similar proportion of patients experience early disease progression, although this proportion is somewhat smaller when bendamustine-based therapy is combined with obinutuzumab.11,32 There are few data on the use of bendamustine plus rituximab (BR) or R-CHOP as second-line therapy for patients with POD24, but parallels may be drawn from unselected patients. One approach to overcoming therapeutic resistance in this setting is using a more aggressive chemotherapy-based sequence. In patients with relapsed FL, after a variety of induction treatments, R-CHOP yielded an 85% overall response rate (ORR), including a 30% CR rate, as defined by CT-based criteria. These patients demonstrated a median PFS of 3 years, improved further by maintenance rituximab.33
Is the patient a candidate for hematopoietic stem cell transplantation?
For fit patients age <65 years without an appropriate clinical trial option, we consider aggressive treatment involving salvage chemoimmunotherapy and consolidative autologous stem cell transplantation (ASCT). This strategy can induce prolonged remissions in FL.31,34 The observation of a plateau in PFS curves suggests cure in a subset, differentiating transplantation from other treatment modalities. Given the consideration of clinical trials and stem cell transplantation at this point in the treatment algorithm (Figure 2), this is often an appropriate time for referral of patients to large academic institutions.
What is the role of high-dose chemotherapy and hematopoietic stem cell transplantation?
The Grupo Español de Linfomas y Trasplantes de Médula Ósea published a retrospective series of ASCT in 655 patients with relapsed FL with nearly 2 decades of follow-up and reported a median OS of 21 years.34 In a subset of 105 patients with POD24 who underwent ASCT in either first CR (CR1) or CR2, 10-year PFS was 58% and 10-year OS was 78%. However, this was a more heterogeneous population, because patients included those with early therapy failure and POD24. Patients underwent transplantation after rescue treatment in CR2 or second partial response, and those in CR1 underwent ASCT for early therapy failure (lack of partial response to frontline therapy).35 In collaboration with the Center for International Blood and Marrow Transplant Research (CIBMTR) and the NLCS, we evaluated whether ASCT improves outcomes in early-relapsing FL in the rituximab era. Patients in the CIBMTR registry experienced POD24 or failure of front-line therapy and underwent ASCT for consolidation. They were compared with outcomes for similar patients in the NLCS who did not undergo ASCT. Patients undergoing ASCT within 1 year of treatment failure had improved 5-year OS compared with those not undergoing ASCT (73% vs 60%; P = .02) and significantly decreased risk of death (HR, 0.63; 95% CI, 0.42-0.94; P = .02).36 Similarly, the German Low Grade Lymphoma Study Group reported outcomes of ASCT in FL patients with early relapse, predominantly in rituximab-naïve patients treated in 2 randomized clinical trials. It too confirmed that ASCT resulted in significantly higher 5-year OS compared with not receiving ASCT (77% vs 46%).37
Use of sibling-matched (MSD) or unrelated-donor allogeneic transplantation has also been studied but has expected toxicity. The CIBMTR separately evaluated unrelated-donor and MSD transplantation compared with ASCT in FL patients with POD24 or refractory disease. Those undergoing MSD allogeneic transplantation had lower relapse rates than those undergoing ASCT (73% vs 70%) and similar OS, but increased nonrelapse mortality (5% vs 17%).38 This may be an option for patients with extensive bone marrow involvement precluding use of ASCT or relapse after ASCT.
Case 1: discussion and outcome
The patient received BR for 6 cycles with PET CR and resolution of hypermetabolic pulmonary nodules and bony lesions. We recommended consolidation with ASCT, given promising survival outcomes in subsets of patients with POD24 who are young and have good PS and few comorbidities. Because the survival benefit with ASCT is greatest when transplantation is performed within the first year of therapy failure, we recommended ASCT now rather than at a later time point. On the basis of his young age and short remission duration, we suggested HLA typing of his 2 siblings should he relapse after ASCT, to allow the future possibility of MSD allogeneic hematopoietic stem cell transplantation if needed.
Case 2
Presentation
A 71-year-old woman with obesity and asthma was diagnosed with grade 2 FL ∼1 year ago after noticing enlarging lymph nodes, fatigue, and night sweats. She had high tumor burden disease, with lymphadenopathy in the bilateral cervical chains, axillae, mediastinum, retroperitoneum, mesentery, and inguinal areas, ranging in size from 2 to 4 cm. She had bone marrow involvement but no other areas of extranodal disease. FLIPI score was 4 (intermediate risk). A PET scan revealed standardized uptake values up to 8. She completed treatment with BR, resulting in PET CR and resolution of her initial symptoms. She was monitored after treatment without maintenance rituximab. She presents 8 months after completing therapy (14 months from diagnosis) with new enlarged axillary and inguinal lymphoma nodes and progressive fatigue. A CT scan demonstrates extensive lymphadenopathy above and below the diaphragm, measuring between 2.5 and 5 cm.
Which targeted therapies are useful for early-relapsing FL?
Immunomodulation
An alternative treatment strategy for early-progressing FL utilizes agents with molecular targets distinct from those of traditional chemoimmunotherapy (Table 2). The immunomodulatory agent lenalidomide has significant activity in relapsed FL. In a randomized phase 2 trial in relapsed FL, 91 patients received either single-agent lenalidomide (range, 15-25 mg) or lenalidomide with rituximab for 12 cycles.39 Overall, treatment was well tolerated, with grade 3 to 4 adverse events including neutropenia in 16% to 20% of patients and fatigue in 9% to 13%. Thrombosis occurred in 16% of patients in the lenalidomide arm. Of the patients receiving lenalidomide, ORR was 53%, with 20% CR. With lenalidomide and rituximab, 76% achieved a response, with 39% achieving CR. Median times to progression of 1.1 and 2.0 years were reported for the 2 arms, respectively.
Efficacy of targeted therapy in early-relapsing FL (POD24)
| Agent . | Overall Response, % . | PFS . | OS . | Reference . |
|---|---|---|---|---|
| Lenalidomide rituximab (n = 43) | 48 | 50% (1 y) | NA | 40 |
| Lenalidomide obinutuzumab (n = 24) | 67 | 75% (1 y) | 87% (1 y) | 41 |
| Idelalisib (n = 37) | 57 | 11 mo (median) | NA | 46 |
| Copanlisib (n = 93)* | 58 | 11 mo (median) | 43 mo (median) | 46,,-49 |
| Agent . | Overall Response, % . | PFS . | OS . | Reference . |
|---|---|---|---|---|
| Lenalidomide rituximab (n = 43) | 48 | 50% (1 y) | NA | 40 |
| Lenalidomide obinutuzumab (n = 24) | 67 | 75% (1 y) | 87% (1 y) | 41 |
| Idelalisib (n = 37) | 57 | 11 mo (median) | NA | 46 |
| Copanlisib (n = 93)* | 58 | 11 mo (median) | 43 mo (median) | 46,,-49 |
NA, not applicable.
FL and marginal zone lymphoma patients.
On the basis of these data, the phase 3 MAGNIFY study is investigating 12 cycles of lenalidomide and rituximab followed by randomization comparing maintenance options. Early results of the induction portion revealed a 1-year PFS rate of 66% for all FL patients enrolled and 50% for those patients experiencing POD24 after rituximab plus chemotherapy.40 Lenalidomide and obinutuzumab for up to 18 cycles was investigated in the phase 2 GALEN trial. Preliminary results in 86 relapsed refractory patients described a 74% ORR, including 44% CR. Of the 24 patients demonstrating POD24 after first-line therapy, ORR and CR rate were similar at 67% and 54% respectively. At 1 year, 75% of these patients were alive and without disease progression.41
PI3K inhibition
Phosphatidylinositol 3-kinase (PI3K) inhibition has also demonstrated significant activity in patients with relapsed FL. Developed to target the B cell–restricted δ isoform, idelalisib was approved for patients who have received 2 prior therapies based on a phase 2 trial in patients refractory to rituximab and alkylating agents. Of 125 indolent lymphoma patients, idelalisib demonstrated an ORR of 57% (56% specifically in FL), with 6% CR.42 Median duration of response was 13 months, and median PFS was 11 months. Concomitant inhibition of PI3K α and γ isoforms with copanlisib and duvelisib has demonstrated similar results in this same double-refractory population.43-45 A post hoc subgroup analysis of the idelalisib-treated patients was performed to evaluate outcomes with early progression after first-line chemoimmunotherapy.46 Of the 37 patients identified, efficacy was similar to that in the larger group, including 57% objective response rate, 14% CR, and median PFS of 11 months. Umbralisib is a PI3Kδ inhibitor with a distinct chemical structure allowing for once-daily dosing. Phase 1 data suggest a similar pattern of response, with a potentially improved safety profile, compared with other PI3Kδ inhibitors. This may be related to increased specificity for the δ isoform and subsequently less perturbation of regulatory T cells.47-49
Anti-CD20 inhibition
Further study with anti-CD20 agents is being pursued, given nonoverlapping toxicity profiles and possible synergistic efficacy. Obinutuzumab is a novel glycol-engineered type 2 anti-CD20 monoclonal antibody demonstrating enhanced antibody-dependent cellular cytotoxicity and phagocytosis as well as increased direct cell death compared with rituximab. The randomized phase 3 GADOLIN study compared obinutuzumab plus bendamustine with single-agent bendamustine in patients with previously treated rituximab-refractory indolent NHL. Having demonstrated median PFS of 29 and 14 months, respectively, obinutuzumab was approved for patients with rituximab-refractory follicular lymphoma and warrants additional study in patients with early progression after rituximab.50,51
Together, these results suggest that targeted agents provide similar efficacy in the population of FL patients demonstrating early progression after chemoimmunotherapy compared with unselected patients with relapsed disease. For patients without clinical trial options and who are not candidates for stem cell transplantation, we recommend use of targeted therapeutics, with primary consideration of lenalidomide, obinutuzumab, or a PI3K inhibitor.
Case 2: discussion and outcome
The patient had an excisional biopsy demonstrating recurrent grade 2 FL. Blood tests including lactate dehydrogenase and complete blood count were normal. A PET scan did not reveal occult areas suggestive of transformation. Bone marrow biopsy showed no evidence of involvement. Her asthma was well controlled, and she continued full-time work. Aside from asthma and obesity, she had no other competing comorbid conditions. Eastern Cooperative Oncology Group PS was 0. The patient was enrolled in the SWOG1608 intergroup clinical trial (NCT03269669) for patients with FL relapsing within 2 years of initial chemoimmunotherapy. The trial is comparing CR rates of additional chemoimmunotherapy (obinutuzumab with CHOP or bendamustine) against obinutuzumab paired with either lenalidomide or umbralisib. The patient was randomly assigned to the umbralisib arm. She has completed 5 cycles of treatment with good tolerance. She is experiencing clinical response, with reduction in size of her lymphadenopathy.
Conclusions and future directions
One in 5 patients with FL will experience early disease recurrence and increased risk of death. The reproducibility and validity of this finding have led to POD24 being considered among the most robust known markers of poor outcome in FL. As such, the National Cancer Institute National Clinical Trials Network recently identified early-relapsing FL as its top priority for FL clinical trial research. Strategies to identify predictors of early relapse, such as the m7-FLIPI and use of GEP, are ongoing, combining molecular and clinical biomarkers. One promising emerging method involves incorporating circulating tumor/cell free DNA. In follicular and other NHLs, high circulating tumor/cell free DNA and high total metabolic tumor volume on PET correlates with shorter PFS.52 This is an example of a marker that could be followed as a surrogate for evolving tumor burden.
Because no treatment has been shown to be superior to another in this setting, clinical trial referral should be prioritized. Outside of a study, data suggest a role for idelalisib and copanlisib, and small subset analyses of prospective studies using lenalidomide are promising. Repeated exposure and cumulative toxicity to novel agents over time should be carefully considered to optimize a patient’s access to future treatments. For the appropriate patient who is younger, with good PS and few comorbidities, consideration should be given to ASCT for consolidation of CR1 or CR2, given the excellent outcomes that may be challenging to surpass with novel treatment approaches.
Research is in progress to understand which patients experiencing early therapy failure have the greatest risk of death, which factors predict response to treatment, and which pathways should be targeted therapeutically. There is also optimism regarding growing opportunities to study biomarkers of FL disease resistance and transformation. We expect these new discoveries can pave a way for risk-adapted approaches that can be applied at the time of diagnosis, change the natural history of follicular lymphoma, and help patients live longer, healthier lives.
Acknowledgments
The authors thank the Wilmot Cancer Institute for support in creating this manuscript.
Authorship
Contribution: C.C. conceived the paper, performed research, and wrote the paper; P.M.B. wrote the paper.
Conflict-of-interest disclosure: C.C. received research funding from Celgene, travel reimbursement from Genentech/Roche, and honoraria from Gilead; P.M.B. has consulted for Pharmacyclics/AbbVie, Gilead, Merck, Celgene, Seattle Genetics, TG Therapeutics, Genentech, and Verastem.
Correspondence: Carla Casulo, University of Rochester Medical Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; e-mail: carla_casulo@urmc.rochester.edu.