Key Points
The risk of RT-induced breast cancer after HL is strongly associated with a PRS for breast cancer in the general population.
A PRS, based on 9 SNPs interacting with RT in the occurrence of breast cancer after HL, also increased RT-induced breast cancer risk.
Abstract
Female Hodgkin lymphoma (HL) patients treated with chest radiotherapy (RT) have a very high risk of breast cancer. The contribution of genetic factors to this risk is unclear. We therefore examined 211 155 germline single-nucleotide polymorphisms (SNPs) for gene-radiation interaction on breast cancer risk in a case-only analysis including 327 breast cancer patients after chest RT for HL and 4671 first primary breast cancer patients. Nine SNPs showed statistically significant interaction with RT on breast cancer risk (false discovery rate, <20%), of which 1 SNP in the PVT1 oncogene attained the Bonferroni threshold for statistical significance. A polygenic risk score (PRS) composed of these SNPs (RT-interaction-PRS) and a previously published breast cancer PRS (BC-PRS) derived in the general population were evaluated in a case-control analysis comprising the 327 chest-irradiated HL patients with breast cancer and 491 chest-irradiated HL patients without breast cancer. Patients in the highest tertile of the RT-interaction-PRS had a 1.6-fold higher breast cancer risk than those in the lowest tertile. Remarkably, we observed a fourfold increased RT-induced breast cancer risk in the highest compared with the lowest decile of the BC-PRS. On a continuous scale, breast cancer risk increased 1.4-fold per standard deviation of the BC-PRS, similar to the effect size found in the general population. This study demonstrates that genetic factors influence breast cancer risk after chest RT for HL. Given the high absolute breast cancer risk in radiation-exposed women, these results can have important implications for the management of current HL survivors and future patients.
Introduction
Women who are treated at young ages with chest radiotherapy (RT) for Hodgkin lymphoma (HL) have a 5 to 20 times increased risk of breast cancer compared with the general population.1-11 The cumulative incidence of breast cancer up to 40 years after treatment with mantle-field RT is 30% to 40%,5,6,10 in the range of risks observed in BRCA1/2 mutation carriers.12 The risk of RT-induced breast cancer rises with increasing radiation dose and volume, but not all female HL survivors treated with high-dose, high-volume RT develop breast cancer. Some variation in risk is explained by age at RT exposure, which is inversely related with breast cancer risk, and premature menopause induced by concomitant alkylating-chemotherapy treatment, which reduces risk.13 However, variation in risk may also be due to genetic factors. The high risk of breast cancer in this population provides an excellent opportunity to investigate the genetic basis for differential sensitivity to radiation carcinogenesis. Although it is well known that ionizing radiation induces DNA damage, the molecular mechanisms underlying radiation-induced breast carcinogenesis are unclear. To date, there is no clear evidence that known high-risk breast cancer susceptibility genes contribute to RT-induced breast cancer risk in HL patients.14-17 However, there may be a more important role for common susceptibility variants, as suggested by genetic association studies in women exposed to low-dose radiation, albeit with conflicting results.18-26 The role of single-nucleotide polymorphisms (SNPs) in breast cancer risk after therapeutic high-dose radiation has been investigated in few studies: a small genome-wide association study (GWAS) on any second solid malignancy in childhood HL survivors27 and a GWAS on radiation-induced breast cancer in childhood cancer survivors.28 In addition, Ma et al investigated 14 SNPs previously associated with breast cancer in the general population in HL survivors.29
In the current study, we used a 2-step design to investigate whether there are subgroups of women exposed to chest RT who are genetically more susceptible to radiation-induced breast cancer. We first used a case-only analysis to evaluate interactions between 211 155 SNPs and chest RT, by comparing patients with breast cancer after chest RT for HL with first primary breast cancer patients previously unexposed to RT. We then conducted a nested breast cancer case-control analysis among chest-irradiated HL survivors to evaluate a polygenic risk score (PRS) composed of RT-interacting SNPs from the case-only analysis (RT-interaction-PRS). As a separate aim, we studied the effect of a previously published breast cancer PRS (BC-PRS)30 in the general population on breast cancer risk among chest-irradiated HL survivors.
Patients and methods
Study design
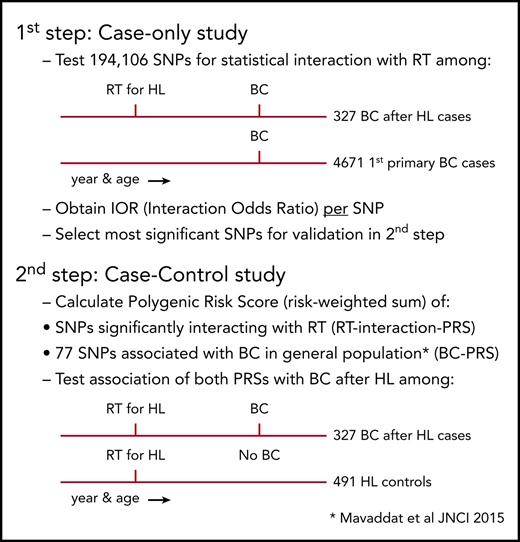
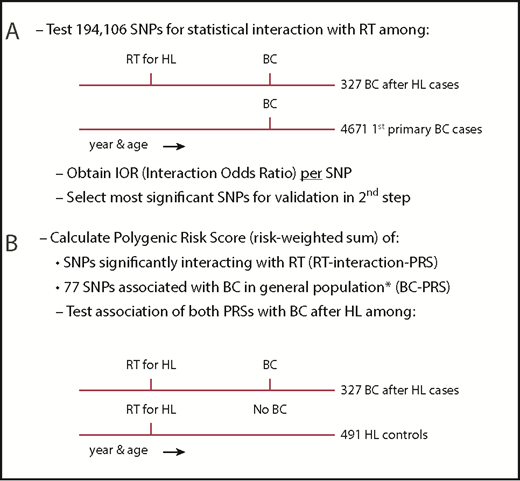
When studying interaction between RT and genetic variation on breast cancer after HL, a classical case-control study nested in a cohort of HL survivors would not be informative because, until recently, 90% of the patients with breast cancer after HL received RT, resulting in too few unexposed cases. Therefore, we used a 2-step design to identify susceptibility variants for radiation-induced breast cancer (Figure 1). First, we examined gene-radiation interaction for 211 155 SNPs in a case-only analysis comparing patients with breast cancer after chest RT for HL (further referred to as breast cancer after HL cases) and first primary breast cancer patients (further referred to as first primary breast cancer cases). For each SNP, we used logistic regression analysis to estimate the per-allele interaction odds ratio (IOR), a measure of departure from a multiplicative joint effect of the SNP and chest RT, for the risk of breast cancer, assuming independence between chest RT and the SNP in women from the general population.31
Study design. (A) First step: case-only study. (B) Second step: case-control study. IOR, interaction odds ratio. *See Mavaddat et al.30
Study design. (A) First step: case-only study. (B) Second step: case-control study. IOR, interaction odds ratio. *See Mavaddat et al.30
Second, we combined interacting SNPs in a PRS, that is, the sum of risk alleles weighted by their effect size (see supplemental Methods A [available on the Blood Web site] for details) and evaluated the association between this PRS and the risk of breast cancer after chest RT in a breast cancer case-control analysis among irradiated HL survivors, using an independent control group of chest-irradiated HL survivors without breast cancer as controls (further referred to as HL controls). We similarly evaluated a second PRS, which was previously reported to be associated with breast cancer in the general population (the BC-PRS).30
Study population and genotyping
For the case-only analysis, we pooled 339 cases with breast cancer after HL from 3 breast cancer case-control studies29,32-34 nested in HL survivor cohorts: the Childhood Cancer Survivor study (CCSS),35 a British HL cohort,10 and the Dutch Hodgkin Lymphoma Cohort.6 Blood samples from these cases were genotyped using a custom Illumina iSelect Array comprising 211 155 SNPs, specially designed for the European Collaborative Oncological Gene-Environment Study (EU-COGS) project (referred to as iCOGS array).36 Extensive patient and HL treatment characteristics, as well as follow-up data, were available from medical records,4,29,35 through questionnaires sent to general practitioners and study participants, and from record linkages with national cancer registries.6,10,14,29,32-34 Female patients with breast cancer after HL were included in our study if they were diagnosed with primary breast cancer >8 years after chest RT for HL before the age of 41 years (see supplemental Methods B for definition of chest RT). Cases with breast cancer after HL were frequency matched (1:∼14) on age and year of breast cancer diagnosis (5-year intervals) and country, to 4673 first primary breast cancer cases of European origin not known to be exposed to chest RT. These were selected from 19 275 participants of 10 studies from The Netherlands, United Kingdom, and the United States within the Breast Cancer Association Consortium (BCAC)36 for whom iCOGS genotype data were available. When there were too few subjects in a specific age category, we oversampled in an adjacent age category in the same calendar year category.
For the case-control analysis, we included the 339 cases with breast cancer after HL mentioned previously and 508 HL survivors treated with chest RT who did not develop breast cancer until end of follow-up, available from the 3 breast cancer case-control studies described earlier in this section. For all HL controls without breast cancer, we collected similar data as described earlier in this section for cases with breast cancer after HL. In the published original case-control studies,13,32-34 which examined radiation dose-response, 1 to 4 controls were individually matched to each case. Controls had to have survived without breast cancer at least as long as the interval between HL and breast cancer for the corresponding case, and in case of the US study, had to have donated a blood sample. In addition, controls had to match the case on age at HL treatment (±3 years) and date of HL treatment (±5 years). Controls from the original case-control studies were excluded if they were not treated with chest RT, were treated at or after age 41 years, and/or did not donate a blood sample. In addition, controls were excluded if they developed breast cancer after the year of breast cancer diagnosis of the case to whom they had been matched. For the current study, we added recently diagnosed breast cancer after HL cases fulfilling the inclusion criteria. All separate studies involved in this collaboration were approved by the relevant institutional review boards, and all individuals gave written informed consent.
Quality control on genotype data
After quality control, 194 106 SNPs measured in 4671 first primary breast cancer cases, 327 cases with breast cancer after HL, and 491 HL controls without breast cancer remained for analyses. See supplemental Methods C for details on quality control.
Statistical analyses
In the case-only analysis, comparing breast cancer after HL cases and first primary breast cancer cases, we estimated the per-allele IOR by unconditional logistic regression analysis for all variants passing quality control, adjusting for the matching factors (age and year of breast cancer diagnosis, both continuous, and country) and the first principal component describing remaining genetic ethnic differences among European subjects (referred to as ethnicity) (see supplemental Methods D). P values for the IORs were calculated by the score test performed using the GenABEL package within R (see supplemental Methods E). Based on a conservative Bonferroni correction, SNPs with a P value < 2.6E-07 were considered statistically significant. Furthermore, we applied the false discovery rate (FDR) by Benjamini and Hochberg37 to identify SNPs among which the expected proportion of false positives is <20% (q value = 0.2). For significant SNPs in linkage disequilibrium (LD; r2 > 0.7), only the SNP with the lowest P value was included in the PRS.
Subsequently, for all subjects in the case-control analysis (breast cancer after HL cases and HL controls), we calculated the RT-interaction-PRS consisting of SNPs interacting with RT on breast cancer at 20% FDR and the 77 SNP BC-PRS. Missing genotypes were imputed by the mode among HL controls. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for RT-induced breast cancer after HL were calculated by unconditional logistic regression per standard deviation (SD) increase in either the RT-interaction-PRS and/or the BC-PRS, adjusted for each other and for age at HL diagnosis (continuous), year of HL diagnosis (4 periods), country, and ethnicity. We also calculated ORs for breast cancer by categories of the PRSs (tertiles for the RT-interaction-PRS and deciles for the BC-PRS). P values for the ORs were based on Wald tests. Interaction between the RT-interaction-PRS and gonadotoxic treatment of HL (yes/no) and between the RT-interaction-PRS and age at HL treatment (≤20/>20 years) was tested by stratification on these factors. As a sensitivity analysis, we assessed the association of a PRS including only the SNPs attaining the Bonferroni threshold for statistical significance. For all Bonferroni-significant SNPs in the case-only analysis, we also tested their individual association with breast cancer after chest RT in the case-control analysis using logistic regression adjusted for age at and year of HL diagnosis, country, and ethnicity. All analyses were conducted with R software (www.r-project.org).
Data availability
Nonidentifiable data that support the findings of this study will be made available upon reasonable request. Access to the BCAC data are governed by the Data Access Coordinating Committee from BCAC. Data from the CCSS study can be retrieved from dbGAP using accession number phs001327.v1.p1.
Results
Study populations of the case-only and case-control analysis
We included 327 breast cancer after HL cases from cohorts of female HL patients in The Netherlands, United Kingdom, and United States and 4671 frequency-matched first primary breast cancer cases previously unexposed to RT from the same countries in the case-only analysis. Furthermore, we included 491 HL controls in the case-control analysis (see Table 1 for the numbers of subjects by country). The median age at breast cancer diagnosis was 45 years (range, 24-76 years) for breast cancer after HL cases and 46 years (range, 22-84 years) for age-matched first primary breast cancer cases (Table 1). The median interval between HL and breast cancer diagnosis was 24 years (range, 9-46 years). For HL controls, median follow-up was 30 years (range, 9-49 years). Most HL cases and controls (87%) were treated with mantle-field irradiation, whereas 11% of the HL cases and controls received mediastinal radiotherapy without axillary node radiotherapy. Approximately one-half of the breast cancer after HL cases and almost 60% of HL controls were treated for HL with chemotherapy in addition to RT. Approximately 45% of breast cancer after HL cases and 57% of HL controls received gonadotoxic treatment (ie, alkylating chemotherapy and/or pelvic RT).
Population characteristics of the breast cancer after HL cases, first primary breast cancer cases, and HL controls without breast cancer
| . | Breast cancer after HL cases, N = 327 . | First primary breast cancer cases, N = 4671 . | HL controls without breast cancer, N = 491 . |
|---|---|---|---|
| Age at breast cancer diagnosis, y | |||
| Median (range) | 45 (24-76) | 46 (22-84) | NA |
| 20-29 | 8 (2.4) | 96 (2.1) | NA |
| 30-39 | 88 (26.9) | 855 (18.3) | NA |
| 40-49 | 139 (42.5) | 2224 (47.6) | NA |
| 50-59 | 68 (20.8) | 1129 (24.2) | NA |
| 60-69 | 20 (6.1) | 314 (6.7) | NA |
| 70+ | 4 (1.2) | 53 (1.1) | NA |
| Year of breast cancer diagnosis* | |||
| Median (range) | 2003 (1984-2013) | 2000 (1964-2011) | NA |
| <1990 | 12 (3.7) | 226 (4.8) | NA |
| 1990-1994 | 25 (7.6) | 606 (13.0) | NA |
| 1995-1999 | 72 (22.0) | 1377 (29.5) | NA |
| 2000-2004 | 86 (26.3) | 1329 (28.5) | NA |
| 2005-2009 | 99 (30.3) | 1067 (22.8) | NA |
| 2010-2014 | 33 (10.1) | 66 (1.4) | NA |
| Age at HL diagnosis, y | |||
| Median (range) | 19 (10-40) | NA | 22 (6-40) |
| <15 | 40 (12.2) | NA | 36 (7.3) |
| 15-19 | 134 (41.0) | NA | 140 (28.5) |
| 20-24 | 76 (23.2) | NA | 140 (28.5) |
| 25-29 | 38 (11.6) | NA | 76 (15.5) |
| 30-34 | 31 (9.5) | NA | 85 (17.3) |
| 35-40 | 8 (2.4) | NA | 14 (2.9) |
| Year of HL diagnosis† | |||
| 1965-1973 | 92 (28.1) | NA | 119 (24.2) |
| 1974-1979 | 120 (36.7) | NA | 132 (26.9) |
| 1980-1984 | 60 (18.3) | NA | 109 (22.2) |
| 1985-1999 | 55 (16.8) | NA | 131 (26.7) |
| Interval between HL and breast cancer diagnosis (cases) or end of follow-up (controls), y | |||
| Median (range) | 24 (9-46) | NA | 30 (9-49) |
| 9-<15 | 28 (8.6) | NA | 6 (1.2) |
| ≥15-<25 | 144 (44.0) | NA | 113 (23.0) |
| ≥25-<35 | 127 (38.8) | NA | 238 (48.5) |
| ≥35 | 28 (8.6) | NA | 134 (27.3) |
| HL treatment‡ | |||
| RT only | 160 (48.9) | NA | 201 (40.9) |
| RT and chemotherapy | 160 (48.9) | NA | 284 (57.8) |
| RT; chemotherapy missing | 7 (2.1) | NA | 6 (1.2) |
| Mantle-field irradiation§ | |||
| Yes | 234 (90.7) | NA | 371 (84.9) |
| No | 18 (7.0) | NA | 60 (13.7) |
| Missing | 6 (2.3) | NA | 6 (1.4) |
| Pelvic RT|| | |||
| Yes | 39 (11.9) | NA | 59 (12.0) |
| No | 288 (88.1) | NA | 432 (88.0) |
| Alkylating chemotherapy¶ | |||
| Yes | 133 (40.7) | NA | 253 (51.5) |
| No | 176 (53.8) | NA | 211 (43.0) |
| Missing | 18 (5.5) | NA | 27 (5.5) |
| Gonadotoxic treatment | |||
| Alkylating chemotherapy and/or pelvic RT | 152 (46.5) | NA | 278 (56.6) |
| No alkylating chemotherapy and no pelvic RT | 158 (48.3) | NA | 192 (39.1) |
| Missing | 17 (5.2) | NA | 21 (4.3) |
| Country | |||
| The Netherlands | 112 (34.3) | 1646 (35.2) | 168 (34.2) |
| United Kingdom | 146 (44.6) | 2380 (51.0) | 269 (54.8) |
| United States | 69 (21.1) | 645 (13.8) | 54 (11.0) |
| . | Breast cancer after HL cases, N = 327 . | First primary breast cancer cases, N = 4671 . | HL controls without breast cancer, N = 491 . |
|---|---|---|---|
| Age at breast cancer diagnosis, y | |||
| Median (range) | 45 (24-76) | 46 (22-84) | NA |
| 20-29 | 8 (2.4) | 96 (2.1) | NA |
| 30-39 | 88 (26.9) | 855 (18.3) | NA |
| 40-49 | 139 (42.5) | 2224 (47.6) | NA |
| 50-59 | 68 (20.8) | 1129 (24.2) | NA |
| 60-69 | 20 (6.1) | 314 (6.7) | NA |
| 70+ | 4 (1.2) | 53 (1.1) | NA |
| Year of breast cancer diagnosis* | |||
| Median (range) | 2003 (1984-2013) | 2000 (1964-2011) | NA |
| <1990 | 12 (3.7) | 226 (4.8) | NA |
| 1990-1994 | 25 (7.6) | 606 (13.0) | NA |
| 1995-1999 | 72 (22.0) | 1377 (29.5) | NA |
| 2000-2004 | 86 (26.3) | 1329 (28.5) | NA |
| 2005-2009 | 99 (30.3) | 1067 (22.8) | NA |
| 2010-2014 | 33 (10.1) | 66 (1.4) | NA |
| Age at HL diagnosis, y | |||
| Median (range) | 19 (10-40) | NA | 22 (6-40) |
| <15 | 40 (12.2) | NA | 36 (7.3) |
| 15-19 | 134 (41.0) | NA | 140 (28.5) |
| 20-24 | 76 (23.2) | NA | 140 (28.5) |
| 25-29 | 38 (11.6) | NA | 76 (15.5) |
| 30-34 | 31 (9.5) | NA | 85 (17.3) |
| 35-40 | 8 (2.4) | NA | 14 (2.9) |
| Year of HL diagnosis† | |||
| 1965-1973 | 92 (28.1) | NA | 119 (24.2) |
| 1974-1979 | 120 (36.7) | NA | 132 (26.9) |
| 1980-1984 | 60 (18.3) | NA | 109 (22.2) |
| 1985-1999 | 55 (16.8) | NA | 131 (26.7) |
| Interval between HL and breast cancer diagnosis (cases) or end of follow-up (controls), y | |||
| Median (range) | 24 (9-46) | NA | 30 (9-49) |
| 9-<15 | 28 (8.6) | NA | 6 (1.2) |
| ≥15-<25 | 144 (44.0) | NA | 113 (23.0) |
| ≥25-<35 | 127 (38.8) | NA | 238 (48.5) |
| ≥35 | 28 (8.6) | NA | 134 (27.3) |
| HL treatment‡ | |||
| RT only | 160 (48.9) | NA | 201 (40.9) |
| RT and chemotherapy | 160 (48.9) | NA | 284 (57.8) |
| RT; chemotherapy missing | 7 (2.1) | NA | 6 (1.2) |
| Mantle-field irradiation§ | |||
| Yes | 234 (90.7) | NA | 371 (84.9) |
| No | 18 (7.0) | NA | 60 (13.7) |
| Missing | 6 (2.3) | NA | 6 (1.4) |
| Pelvic RT|| | |||
| Yes | 39 (11.9) | NA | 59 (12.0) |
| No | 288 (88.1) | NA | 432 (88.0) |
| Alkylating chemotherapy¶ | |||
| Yes | 133 (40.7) | NA | 253 (51.5) |
| No | 176 (53.8) | NA | 211 (43.0) |
| Missing | 18 (5.5) | NA | 27 (5.5) |
| Gonadotoxic treatment | |||
| Alkylating chemotherapy and/or pelvic RT | 152 (46.5) | NA | 278 (56.6) |
| No alkylating chemotherapy and no pelvic RT | 158 (48.3) | NA | 192 (39.1) |
| Missing | 17 (5.2) | NA | 21 (4.3) |
| Country | |||
| The Netherlands | 112 (34.3) | 1646 (35.2) | 168 (34.2) |
| United Kingdom | 146 (44.6) | 2380 (51.0) | 269 (54.8) |
| United States | 69 (21.1) | 645 (13.8) | 54 (11.0) |
Data are n (%) unless otherwise noted.
NA, not applicable.
Four cases with breast cancer after HL had missing year of breast cancer diagnosis, which were imputed with the median year of breast cancer diagnosis among participants from the same country.
Four cases with breast cancer after HL and 6 HL controls had missing year of HL diagnosis. These missing years were imputed with the median year of HL diagnosis among participants in the same group (cases or controls) from the same country.
For the Dutch HL survivors, chest RT was defined as (in)complete mantle field or mediastinal RT, or RT to the lungs or axilla. Subjects with only infradiaphragmatic RT were excluded. For HL survivors from the United States, chest RT was defined as chest or total nodal RT (subjects with only brain, other head, neck, abdomen, spine, pelvis, and/or limb RT were excluded). For HL survivors from the United Kingdom, chest RT was defined as mantle field, chest, mediastinal, axillary, mini mantle field or partial chest RT (subjects with only neck, clavicular and/or head or other supradiaphragmatic RT or infradiaphragmatic RT, RT field unknown or chemotherapy only were excluded).
Information on the radiation fields was only available for HL survivors from the United Kingdom and The Netherlands.
Pelvic RT encompassed RT to the whole abdomen or iliac nodes on both sides, or RT with inverted Y field, in women with no (successful) oophoropexy.
Alkylating chemotherapy consists of combinations of cytostatic agents with at least 1 alkylating agent (ie, procarbazine, cyclophosphamide, ifosfamide, lomustine, melphalan, dacarbazine, cisplatin, mechlorethamine, chlorambucil, and carmustine).
SNPs interacting with RT on breast cancer risk (case-only analysis)
We tested 194 106 SNPs that passed quality control for an interaction with chest RT in the case-only analysis of breast cancer patients (QQ plot is depicted in supplemental Figure 1). As shown in Table 2, 3 SNPs were statistically significantly associated at the Bonferroni threshold for multiple testing (P < 2.6E-07) and 7 additional SNPs met the 20% FDR threshold, of which 1 SNP was excluded because of strong LD (r2 = 0.9). The estimated per-allele IORs for these 9 SNPs ranged from 1.6 to 2.2. Most SNPs were quite common in the breast cancer after HL cases with minor allele frequencies (MAFs) between 2.8% and 43.7%.
Characteristics of SNPs statistically significantly (20% FDR) interacting with RT in the case-only analysis
| SNP . | Locus . | Chr . | Position* . | Alleles . | Breast cancer after HL cases, N = 327 . | First primary breast cancer cases, N = 4671 . | Statistical interaction with chest RT on breast cancer risk† . | Weight RT-interaction-PRS . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF . | N called . | MAF . | N called . | IOR . | 95% CI . | P‡ . | Log IOR . | |||||
| rs10505506 | PVT1 | 8 | 129114473 | G/C | 0.407 | 327 | 0.306 | 4670 | 1.6 | 1.3-1.8 | 3.1E-08 | 0.44 |
| rs12086369 | 1p31.1 | 1 | 79644149 | G/A | 0.073 | 324 | 0.035 | 4667 | 2.1 | 1.5-2.8 | 9.4E-08 | 0.74 |
| rs9461776 | HLA | 6 | 32683713 | A/G | 0.133 | 327 | 0.079 | 4671 | 1.8 | 1.4-2.3 | 1.1E-07 | 0.59 |
| MitoA7769G | MT | 7769 | A/G | 0.052 | 325 | 0.020 | 4653 | 2.1 | 1.5-3.0 | 2.8E-06 | 0.76 | |
| rs1017639 | CPT1A | 11 | 68355110 | A/C | 0.073 | 327 | 0.043 | 4669 | 1.9 | 1.4-2.6 | 2.8E-06 | 0.63 |
| MitoT9900C | MT | 9900 | A/G | 0.028 | 325 | 0.011 | 4669 | 2.0 | 1.3-3.2 | 3.7E-06 | 0.71 | |
| MitoA13781G | MT | 13781 | A/G | 0.036 | 306 | 0.011 | 4592 | 2.2 | 1.5-3.3 | 4.3E-06 | 0.80 | |
| rs2296008 | COL19A1 | 6 | 70935424 | G/A | 0.041 | 327 | 0.020 | 4669 | 2.2 | 1.4-3.4 | 6.8E-06 | 0.79 |
| rs3815871 | PVT1 | 8 | 129077760 | G/C | 0.437 | 327 | 0.341 | 4671 | 1.5 | 1.3-1.8 | 8.5E-06 | 0.40 |
| SNP . | Locus . | Chr . | Position* . | Alleles . | Breast cancer after HL cases, N = 327 . | First primary breast cancer cases, N = 4671 . | Statistical interaction with chest RT on breast cancer risk† . | Weight RT-interaction-PRS . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF . | N called . | MAF . | N called . | IOR . | 95% CI . | P‡ . | Log IOR . | |||||
| rs10505506 | PVT1 | 8 | 129114473 | G/C | 0.407 | 327 | 0.306 | 4670 | 1.6 | 1.3-1.8 | 3.1E-08 | 0.44 |
| rs12086369 | 1p31.1 | 1 | 79644149 | G/A | 0.073 | 324 | 0.035 | 4667 | 2.1 | 1.5-2.8 | 9.4E-08 | 0.74 |
| rs9461776 | HLA | 6 | 32683713 | A/G | 0.133 | 327 | 0.079 | 4671 | 1.8 | 1.4-2.3 | 1.1E-07 | 0.59 |
| MitoA7769G | MT | 7769 | A/G | 0.052 | 325 | 0.020 | 4653 | 2.1 | 1.5-3.0 | 2.8E-06 | 0.76 | |
| rs1017639 | CPT1A | 11 | 68355110 | A/C | 0.073 | 327 | 0.043 | 4669 | 1.9 | 1.4-2.6 | 2.8E-06 | 0.63 |
| MitoT9900C | MT | 9900 | A/G | 0.028 | 325 | 0.011 | 4669 | 2.0 | 1.3-3.2 | 3.7E-06 | 0.71 | |
| MitoA13781G | MT | 13781 | A/G | 0.036 | 306 | 0.011 | 4592 | 2.2 | 1.5-3.3 | 4.3E-06 | 0.80 | |
| rs2296008 | COL19A1 | 6 | 70935424 | G/A | 0.041 | 327 | 0.020 | 4669 | 2.2 | 1.4-3.4 | 6.8E-06 | 0.79 |
| rs3815871 | PVT1 | 8 | 129077760 | G/C | 0.437 | 327 | 0.341 | 4671 | 1.5 | 1.3-1.8 | 8.5E-06 | 0.40 |
Chr, chromosome; CI, confidence interval; FDR, false discovery rate; IOR, interaction odds ratio; MAF, minor allele frequency; MT, mitochondrial DNA.
Positions are based on NCBI36/hg18.
Logistic regression analysis per SNP to test the log additive effect per allele (per-allele IOR) with adjustment for age at and year of breast cancer diagnosis, country, and ethnicity.
All listed SNPs were significant at a 20% FDR. Top 3 SNPs were statistically significant at the Bonferroni threshold (P < 2.6E-07).
PRS for RT-induced breast cancer (case-control analysis)
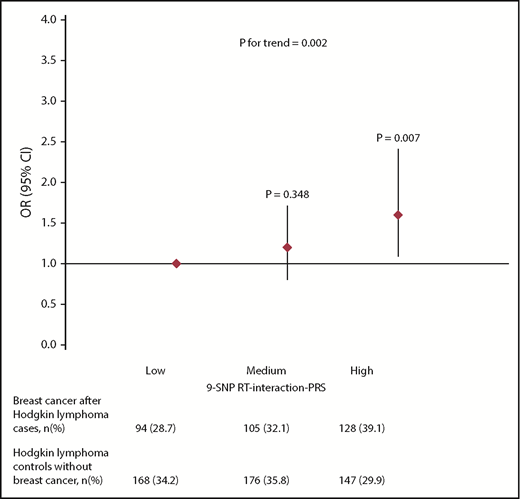
We constructed an RT-interaction-PRS of the 9 SNPs that showed a statistically significant (FDR, 20%) interaction with RT-induced breast cancer. The RT-interaction-PRS increased breast cancer risk after chest RT for HL with ORs of 1.2 (95% CI, 0.8-1.7; P = .348) and 1.6 (95% CI, 1.1-2.4; P = .007), respectively, for the middle and highest tertiles compared with the lowest tertile, adjusted for age and year of HL diagnosis, country, ethnicity, and the BC-PRS (Figure 2; supplemental Table 1). The OR per 1 SD of the RT-interaction-PRS was 1.3 (95% CI, 1.1-1.5; P = .002). Additional adjustment for gonadotoxic treatment did not affect the association of the RT-interaction-PRS with breast cancer risk (ORadjusted, 1.3; 95% CI, 1.1-1.5), suggesting that it is unlikely that chemotherapy has confounded our analyses. In addition, stratified analyses resulted in similar associations between the RT-interaction-PRS and breast cancer risk among women who received gonadotoxic treatment (alkylating chemotherapy and/or pelvic RT) and women who did not; we observed no statistically significant interaction between the RT-interaction-PRS and gonadotoxic treatment (P = .337; supplemental Table 1). Likewise, stratification by age at HL treatment (≤20, >20 years) did not result in different associations between the RT-interaction-PRS and breast cancer risk after RT for HL; there was no interaction between age at HL treatment and the RT-interaction PRS (P = .954).
Risk of breast cancer after chest RT by tertiles of the RT-interaction-PRS among HL survivors.
Risk of breast cancer after chest RT by tertiles of the RT-interaction-PRS among HL survivors.
In a sensitivity analysis, we observed that a PRS containing only the 3 SNPs reaching the Bonferroni threshold for statistical significance also increased breast cancer risk with ORs of 1.4 (95% CI, 1.0-2.1; P = .070) and 1.6 (95% CI, 1.1-2.2; P = .018), respectively, for the middle and highest tertile compared with the lowest tertile which consisted of noncarriers. The OR per 1 SD of the 3 SNP RT-interaction-PRS was 1.2 (95% CI, 1.0-1.4; P = .014).
To confirm the observed associations, we also evaluated the individual effects of the 3 Bonferroni-significant SNPs on RT-induced breast cancer in the case-control analysis among chest-irradiated HL survivors (supplemental Table 2). Of these, an intronic variant in oncogene PVT1 (rs10505506) was associated with RT-induced breast cancer risk after HL with an OR of 1.3 (95% CI, 1.1-1.6; P = .007) per allele copy. Of note, rs10505506 is not in LD (r2 < 0.3 in Europeans from the 1000 Genomes Project38 ) with previously identified cancer risk variants in the PVT1 locus (supplemental Figure 2).
PRS based on known breast cancer SNPs (case-control analysis)
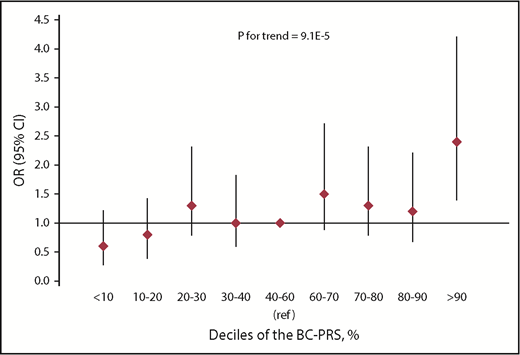
To evaluate the combined effect of known breast cancer SNPs, we studied a BC-PRS containing 76 SNPs that increase breast cancer risk in the general population30 in chest-irradiated HL survivors. The BC-PRS was associated with a 1.4-fold increased risk of RT-induced breast cancer (95% CI, 1.2-1.6; P = 9.1E-05) per SD increase in the BC-PRS. The ORs for developing breast cancer after chest RT for HL by deciles of the BC-PRS, compared with women in the middle quintile (40th to 60th percentile), are shown in Figure 3 and supplemental Table 3. The 10% of women with the lowest BC-PRS had an OR of 0.6 (95% CI, 0.3-1.1; P = .133) for developing RT-induced breast cancer compared with women in the middle quintile, whereas the OR for the 10% of women with the highest BC-PRS was 2.4 (95% CI, 1.4-4.2; P = .002), adjusted for age and year of HL diagnosis, country, ethnicity, and the RT-interaction-PRS (in tertiles). This results in a fourfold relative risk for the 10% of women with the highest compared with the lowest BC-PRS. There was no interaction between the RT-interaction-PRS and the BC-PRS (P = .645).
Risk of breast cancer after chest RT by deciles of the BC-PRS in the breast cancer after HL case-control analysis.
Risk of breast cancer after chest RT by deciles of the BC-PRS in the breast cancer after HL case-control analysis.
Discussion
This study demonstrates that genetic factors influence the risk of breast cancer after chest RT for HL. We showed that a BC-PRS, consisting of 77 SNPs previously associated with breast cancer in the general population, also substantially increases the risk of breast cancer in chest-irradiated HL survivors. In addition, we identified 9 SNPs interacting with chest RT and the risk of breast cancer after HL and we showed a statistically significant association of a PRS composed of these interaction SNPs with breast cancer risk after chest RT for HL using an independent control group. These results imply that the absolute risk of breast cancer due to irradiation would be (even) larger among women at high genetic risk, which is relevant for clinical risk prediction.
Importantly, we validated the previously published BC-PRS in a high-risk population of female chest-irradiated HL survivors and found that there are large differences in risk between women with a low and high PRS. More specifically, we observed a fourfold increased relative risk between chest-irradiated HL survivors in the highest compared with the lowest decile of the BC-PRS. On a continuous scale, the effect size was very similar to that found in the general population (OR of 1.4 per SD in our study of HL survivors compared with ORs of 1.4 to 1.6 per SD in the general population).30,39 These results indicate that the effects of radiation exposure and common susceptibility variants, summarized in the PRS, combine approximately multiplicatively. Given the high absolute breast cancer risk in radiation-exposed women, these results have important implications for their management. The BC-PRS can be used to help guide treatment decisions in newly diagnosed HL patients as well as to help determine breast surveillance strategies for irradiated HL survivors. Annual breast cancer surveillance between the ages of 25 and 50 years is currently recommended by the International Late Effects of Childhood Cancer Guideline Harmonization Group for female survivors of childhood, adolescent, and young adult cancer who received ≥20 Gy chest radiation before age 30 years.40 Less clear is the evidence for surveillance in women treated at older ages, with lower dosages, or with different radiation volumes. Therefore, clinical prediction models for breast cancer that include both clinical and genetic factors can help to identify (additional) women who may benefit from breast cancer surveillance.
We chose to evaluate the 77 SNP BC-PRS by Mavaddat et al30 as this PRS has been associated with breast cancer risk in the general population and in high-risk groups such as BRCA1 and BRCA2 mutation carriers,39,41 allowing direct comparison of the reported effect sizes. Nevertheless, many more common susceptibility variants have recently been identified for breast cancer in the general population.42,43 Addition of these SNPs to the BC-PRS may further improve risk stratification for breast cancer in chest-irradiated HL survivors and in other high-risk groups. Inclusion of SNPs associated with hormone receptor-negative breast cancer may be of particular interest, as several studies have reported that HL survivors are more likely to develop hormone receptor-negative disease.44-46
We applied an innovative design to examine the role of SNP-radiation interactions in breast cancer risk after HL. This is not feasible in a classical breast cancer case-control study in HL survivors, as, until recently, ∼90% of breast cancer cases after HL received chest RT. Therefore, we first performed a case-only analysis in breast cancer patients previously exposed and unexposed to chest RT, followed by a case-control analysis in HL survivors to evaluate the combined effect of the identified RT-interaction SNPs in a PRS. We used a 20% FDR as a cutoff to select SNPs interacting with RT for the RT-interaction-PRS, as it has been shown that the performance of a PRS improves when using more liberal thresholds than the conservative Bonferroni threshold.47,48 Although a PRS consisting of 3 SNPs statistically significant at the Bonferroni threshold showed a similar association with RT-induced breast cancer risk among HL survivors, the goodness of fit was better in the full PRS (data not shown).
The IORs that we estimated in the case-only analysis measure departure from a multiplicative joint effect of chest RT and the SNP, assuming independence between chest RT and the SNP in women from the general population.49 This assumption is likely to be justified except for SNPs associated with HL. SNPs associated with HL may also have shown a significant IOR in the case-only analysis. On the other hand, such SNPs may be associated with both HL and (radiation-induced) breast cancer and, therefore, we did not exclude SNPs previously associated with HL from inclusion in the RT-interaction-PRS. If they were only associated with HL they would have attenuated the association of the RT-interaction-PRS with breast cancer after chest RT in the case-control analysis. In the case-only analysis, we identified 1 SNP (rs9461776) interacting with radiation at 20% FDR significance located in the HLA region, which has extensively been reported to be associated with HL.50 rs9461776 showed no evidence of an association with breast cancer after chest RT (OR, 1.0; 95% CI, 0.8-1.4; P > .5) in the case-control analysis in HL survivors and may therefore have attenuated the association of the RT-interaction-PRS with the risk of breast cancer after chest RT.
Of the 9 SNPs (MAF > 1%) interacting with RT on breast cancer risk at 20% FDR, 1 attained the genome-wide level (P < 5 × 10−8) of statistical significance. This SNP (rs10505506) was also associated with breast cancer risk in chest-irradiated HL survivors (OR, 1.3; 95% CI, 1.1-1.6; P = .007). SNP rs10505506 is located in the intronic region of PVT1, which is a known oncogene regulated by tumor-suppressor p53 encoding a long noncoding RNA and several microRNAs.51,52 PVT1 has been shown to interact with the adjacent proto-oncogene MYC and translocations in this locus have been associated with Burkitt lymphoma. In addition, overexpression of PVT1 is associated with several types of cancers including breast cancer, acute myeloid leukemia, and HL. Likewise, GWAS studies have identified several conditionally independent SNPs in this locus associated with cancer, including breast cancer and HL,53,54 but none of these are in LD with rs10505506. A potential link with radiation has recently been suggested in a mouse model after whole-body irradiation.55
The association of the RT-interaction-PRS with breast cancer risk after HL was not weakened in “low-risk” groups of women irradiated at older age (ie, 20 years or older) or women treated with gonadotoxic treatment. In addition, we did not observe interaction between the RT-interaction-PRS and either gonadotoxic treatment or age at HL treatment. This is in line with the notion that gonadotoxic treatment and age at HL treatment are independent risk factors for breast cancer risk after HL. This suggests that age and treatment-related risk factors for breast cancer after HL and the genetic risk scores (both the RT-interaction-PRS and the BC-PRS) combine multiplicatively as has previously been shown for several reproductive risk factors and the 77 SNP BC-PRS in the general population.56
A limitation of this study is that the study populations for the construction and evaluation of the RT-interaction-PRS were not independent, as the breast cancer after HL cases were included in both analyses. External validation of the RT-interaction-PRS in an independent study is therefore needed to confirm our findings. In addition, we excluded SNPs with a low MAF (<1%) from our analyses, as these low-frequency SNPs are more prone to genotyping errors. However, Morton et al recently reported 2 suggestive associations for low-frequency variants at 11q23 and 1q32.3, both not present on the iCOGS array, with breast cancer risk after childhood cancer,28 suggesting a potential role for low-frequency SNPs in RT-induced breast cancer. Inclusion of these SNPs to the RT-interaction-PRS might strengthen its association with RT-induced breast cancer. Likewise, additional SNPs interacting with RT on breast cancer may be identified when assessing SNP data from denser genotyping chips imputed to a reference panel. However, in this first analysis, we focused on high-quality SNPs specifically selected for the iCOGS array.
In conclusion, we showed that a BC-PRS previously developed in the general population also applies in a high-risk breast cancer population of chest-irradiated HL survivors. In addition, we developed an RT-interaction-PRS composed of 9 SNPs interacting with radiation that was associated with raised breast cancer risk after chest RT for HL. Although our RT-interaction-PRS needs validation in an independent sample, the BC-PRS can already be applied in clinical practice. This can benefit treatment decision-making in future HL patients as well as identification of high-risk survivors eligible for breast cancer surveillance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
For the Dutch Hodgkin Lymphoma cohort, the authors thank Ph. M. P. Poortmans from Elisabeth Hospital Tilburg at the time of the study, M. L. M. Lybeert from Catherina Hospital Eindhoven, G. W. Imhoff from Groningen University Medical Center, J. M. M. Raemaekers from Rijnstate Hospital/Radboud University Medical Center Nijmegen at the time of the study, J. M. Zijlstra from VU Medical Center Amsterdam, and J. H. Borger from Maastricht University Medical Center for inclusion of HL patients. The UK Hodgkin Lymphoma cohort thanks the study participants, study staff, and clinicians who participated in the study as listed in the authorship and acknowledgments in Cooke et al.32
This work was supported by Dutch Cancer Society grant 2010-4720 (F.E.v.L.). The UK Hodgkin Lymphoma cohort was supported by Breast Cancer Now and the European Commission. The Institute of Cancer Research acknowledges funding from the National Institute for Health Research to the Biomedical Research Centre. The Childhood Cancer Survivor study was supported by the National Institutes of Health, National Cancer Institute (CA55727 [principal investigator: G. T. Armstrong]). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765 [principal investigator: C. Roberts]) and the American Lebanese-Syrian Associated Charities (ALSAC).
All sponsors of this work are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: A.W.J.O.-v.W., M.H., M.K.S., H.G.d.H., and F.E.v.L. performed the analyses, interpreted the data, and drafted the manuscript; A.W.J.O.-v.W., H.G.d.H., F.H.v.d.B., M.H., and M.K.S. performed statistical analysis; F.E.v.L. N.S.R., C.P.M.J., A.D.G.K., J.D., H.A.-C., C.A.H, E.J.S., A.C., P.D., M.J.H., J.P., F.J.C., P.P., N.O., D.F.E., B.M.P.A., L.C.S., S.B., R.C., L.L.R., and A.J.S. contributed in the inclusion of patients and data collection; H.G.d.H., M.L.D.B., and A.M.v.E. provided administrative support and coordinated the work; M.H., M.K.S., A.B., and F.E.v.L. supervised the study; and all authors critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Flora E. van Leeuwen, Department of Epidemiology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: f.v.leeuwen@nki.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal