In this issue of Blood, Opstal-van Winden and colleagues report the results of a genome-wide association study to identify constitutional genetic variants (single nucleotide polymorphisms; SNPs) associated with risk of developing radiation-induced breast cancer in Hodgkin lymphoma survivors.1 Therapy-induced cancer is a potentially lethal complication of treatment of a first primary cancer, and breast cancer is one of the most common therapy-induced cancers in long-term survivors of Hodgkin lymphoma treated with radiotherapy. Travis et al2 estimated the cumulative absolute risks of breast cancer following a ≥40 Gy dose at age 25 to be 11% and 29% at 20 and 30 years, respectively. Data from a large cancer registry showed relative risk of breast cancer to be ∼6 times higher in Hodgkin lymphoma survivors compared with the general population.3 As such, the prospective identification of individuals at high risk of radiogenic breast cancer could facilitate improvements in the clinical management of Hodgkin lymphoma patients, reducing subsequent cancer risk and improving outcomes.
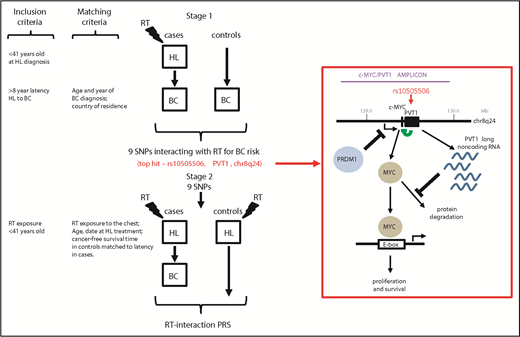
Study design used to develop a PRS for radiogenic breast cancer. Opstal-van Winden and colleagues used a 2-stage approach to identify SNPs interacting with radiotherapy (RT) for breast cancer (BC) risk (top) and subsequently used these to develop a PRS for radiogenic breast cancer in Hodgkin lymphoma (HL) survivors (bottom). Inclusion and matching criteria (left) were used to maximize the power to identify genetic variants associated with risk of radiogenic breast cancer. A SNP (rs10505506) localized to PVT1 significantly associates with risk of radiogenic breast cancer in Hodgkin lymphoma survivors (boxed in red). The PVT1 long noncoding RNA negatively regulates MYC-driven oncogenic transcriptional activity by inhibiting protein degradation. The PVT1 promoter also negatively regulates MYC transcription directly (green). MYC expression drives transcription of downstream target genes via binding to E-box elements in promoters, promoting tumor cell proliferation and survival. c-MYC and PVT1 are coamplified in primary breast cells exposed to ionizing radiation (purple bar shows approximate location of amplicon identified by Wade and colleagues6 ). A risk allele for radiogenic cancer in Hodgkin lymphoma survivors was identified in the PRDM1 gene.5 PRDM1 is a negative regulator of c-MYC transcription, but PRDM1 upregulation in response to ionizing radiation is attenuated in cells carrying the risk variant, leading to elevated c-MYC expression and acquisition of a pro-proliferative phenotype. Additional work is required to determine functionality of the rs10505506 variant, although it is plausible this also operates via the MYC pathway. Approximate amplicon, gene, and SNP locations are based on GRCh37.
Study design used to develop a PRS for radiogenic breast cancer. Opstal-van Winden and colleagues used a 2-stage approach to identify SNPs interacting with radiotherapy (RT) for breast cancer (BC) risk (top) and subsequently used these to develop a PRS for radiogenic breast cancer in Hodgkin lymphoma (HL) survivors (bottom). Inclusion and matching criteria (left) were used to maximize the power to identify genetic variants associated with risk of radiogenic breast cancer. A SNP (rs10505506) localized to PVT1 significantly associates with risk of radiogenic breast cancer in Hodgkin lymphoma survivors (boxed in red). The PVT1 long noncoding RNA negatively regulates MYC-driven oncogenic transcriptional activity by inhibiting protein degradation. The PVT1 promoter also negatively regulates MYC transcription directly (green). MYC expression drives transcription of downstream target genes via binding to E-box elements in promoters, promoting tumor cell proliferation and survival. c-MYC and PVT1 are coamplified in primary breast cells exposed to ionizing radiation (purple bar shows approximate location of amplicon identified by Wade and colleagues6 ). A risk allele for radiogenic cancer in Hodgkin lymphoma survivors was identified in the PRDM1 gene.5 PRDM1 is a negative regulator of c-MYC transcription, but PRDM1 upregulation in response to ionizing radiation is attenuated in cells carrying the risk variant, leading to elevated c-MYC expression and acquisition of a pro-proliferative phenotype. Additional work is required to determine functionality of the rs10505506 variant, although it is plausible this also operates via the MYC pathway. Approximate amplicon, gene, and SNP locations are based on GRCh37.
Numerous patient- and exposure-related factors modify radiogenic breast cancer risk, including age at exposure, cumulative radiation dose, radiation field size, radiation field location (mediastinal or mantle), and early menopause.4 Evidence also suggests a role for constitutional genetics as a determinant of individual risk,5 with the prevailing model suggesting that the genetic contribution to radiogenic breast cancer risk is polygenic and determined by coinheritance of multiple low-penetrance genetic variants in numerous genes.
Based on this premise, Opstal-van Winden and colleagues used an innovative 2-phase approach to identify genetic variants associated with risk of radiogenic breast cancer specifically in Hodgkin lymphoma survivors. Their approach was designed to first identify variants interacting with radiation for breast cancer risk and then use these to generate a polygenic risk score (PRS) for breast cancer in Hodgkin lymphoma survivors while simultaneously eliminating variants associated with Hodgkin lymphoma risk (see figure). Carefully defined inclusion criteria were used to maximize the frequency of likely radiogenic cases while simultaneously minimizing the frequency of “sporadic” second primary breast cancer cases without a radiation etiology. Specifically, the authors restricted their study to cases exposed to radiation at a young age and with a latency of at least 8 years between lymphoma and subsequent breast cancer (see figure). Furthermore, the use of stringent case-control matching was designed to minimize the contribution of established risk factors (such as age at exposure) while simultaneously maximizing the power to discern genetic variants associated with risk of radiogenic breast cancer. This approach was used to generate a PRS based on 9 SNPs that showed a statistically significant interaction with radiotherapy for breast cancer risk. Critically, the PRS retained predictive power irrespective of exposure to gonadotoxic therapy, despite this not being included in the matching criteria. Radiation exposure to the ovaries or systemic alkylating chemotherapy can ablate ovarian function, can reduce estrogen production, and is associated with a significant reduction in radiogenic breast cancer risk in Hodgkin lymphoma survivors.4 Likewise, the PRS retained predictive power irrespective of age at radiation exposure, suggesting applicability to all Hodgkin lymphoma survivors. The study by Opstal-van Winden and colleagues focused on “common” SNPs with a minor allele frequency of >1% in the general population. It is likely that rarer SNPs also contribute to individual risk of radiogenic breast cancer, but identification will require large studies that either directly genotype these variants (using next-generation sequencing, for example) or that use accurate methods for imputing nongenotyped low-frequency variants. Large studies with enhanced statistical power will also be required to discern gene-gene interactions and to overcome stringent statistical thresholds associated with multiple testing in genome-wide studies.
As well as predicting individual risk, the identification of susceptibility variants informs on the molecular mechanisms driving the development of radiogenic cancer. In this regard, it is noteworthy that the most statistically significant of the 9 SNPs identified by Opstal-van Winden and colleagues localizes to the PVT1 long noncoding RNA gene on chromosome 8q24 (rs10505506), adjacent to the c-MYC proto-oncogene (see figure). High-level somatic copy number gain of this locus, capturing both c-MYC and PVT1, is significantly more common in radiogenic breast cancer compared with disease without a radiation etiology.6,7 Furthermore, focal high-level amplification of this locus was reported in primary breast epithelial cells and an immortalized nontransformed breast cell line following exposure to ionizing radiation,6 suggesting c-MYC/PVT1 amplification to be an early somatic event in radiation-driven breast transformation. c-MYC and PVT1 are almost always amplified together in human tumors, and the PVT1 long noncoding RNA was recently identified as a key regular of MYC-driven oncogenic transcriptional activity by augmenting protein stability8 (see figure). There is also evidence that the PVT1 promoter directly regulates MYC transcription, independent of long noncoding RNA expression.9 Knockout of PVT1 reduces MYC protein levels and attenuates tumorigenic potential of cells.8 Other data also suggest a role for dysregulated MYC in radiation-induced breast transformation. Specifically, Best et al5 identified a SNP in the PRDM1 gene associated with radiogenic cancer risk (predominantly breast cancer) in pediatric Hodgkin lymphoma survivors. PRDM1 is a negative regulator of c-MYC transcription, but PRDM1 upregulation in response to ionizing radiation is attenuated in cells carrying the risk variant, leading to elevated c-MYC expression and acquisition of a pro-proliferative phenotype.5 Collectively, these studies provide compelling evidence that dysregulated MYC is a common feature of radiogenic breast cancer during the early stages of transformation. As such, it is plausible that the PVT1 SNP identified by Opstal-van Winden et al also operates via MYC to affect risk of radiogenic breast cancer, although additional work is required to determine functionality of the rs10505506 variant.
Pending independent validation, the data presented by Opstal-van Winden and others5 could aid the development of personalized risk-adapted strategies for the clinical management of Hodgkin lymphoma patients, including alternative treatments and posttherapy surveillance for therapy-induced breast cancer. Such approaches could prove important in pediatric and young adult Hodgkin lymphoma patients where the risk of radiogenic breast cancer is particularly high and associated with premature death.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal