Abstract
Myelodysplastic syndrome (MDS) is characterized by bone marrow failure and a strong propensity for leukemic evolution. Somatic mutations are critical early drivers of the disorder, but the factors enabling the emergence, selection, and subsequent leukemic evolution of these “leukemia-poised” clones remain incompletely understood. Emerging data point at the mesenchymal niche as a critical contributor to disease initiation and evolution. Disrupted inflammatory signaling from niche cells may facilitate the occurrence of somatic mutations, their selection, and subsequent clonal expansion. This review summarizes the current concepts about “niche-facilitated” bone marrow failure and leukemic evolution, their underlying molecular mechanisms, and clinical implications for future innovative therapeutic targeting of the niche in MDS.
MDS: a disease of genetic events in HSCs, but what is missing?
Myelodysplastic syndrome (MDS) is a clonal disorder of hematopoiesis that is characterized by bone marrow failure (BM) and the propensity for evolution toward acute myeloid leukemia (AML). The disorder is driven by (epi)genetic events in hematopoietic stem and/or progenitor cells (HSPCs).1 Emerging insights have revealed that acquisition and selection of “poising” mutations in epigenetic regulators, such as DNMT3A, TET2, ASXL1, and splice factors, are critical first steps in the pathogenesis of MDS.2 HSPCs in MDS typically acquire genetic lesions that, in concert, drive malignant transformation. It remains incompletely understood how a single hematopoietic stem cell (HSC) that is generally considered to be in a predominantly quiescent state acquires multiple mutations. Also, it is not immediately apparent why some “founding” mutations, in genes like ASXL1 and splice factors, that do not result in a cell-intrinsic competitive advantage over nonmutated HSPCs persist in the BM and eventually result in clonal outgrowth.3-6 Finally, it is not immediately clear how relatively minor mutated clones, with consequently considerable remaining residual normal hematopoiesis, result in cytopenia in MDS. In obtaining insight into these open questions, Darwinian evolution principles predict that a cell’s direct environment, or HSPC niche, may play critical roles in the functional competence of HSPCs and the emergence and selection of genetic clones within a population.
A role for the niche? Circumstantial support for a role for the niche in MDS pathogenesis
MDS has long been considered a hematopoietic cell–autonomous disorder in which disease initiation and progression are exclusively driven by hematopoietic cell–intrinsic genetic events. However, several earlier observations have challenged this reductionist view and suggested a contribution of the BM microenvironment to disease pathogenesis. Bone abnormalities suggesting “adynamic” bone, characterized by reduced osteoblast numbers, decreased mineral apposition rates, and osteoporosis, have been noted in MDS patients in comparison with age-matched controls.7,8 Early histologic findings pointed to the disruption of the BM architecture as a common finding in MDS (see Raaijmakers9 for additional details). BM-derived stromal cells (BMDSCs), a readily available source of stromal cells from the hematopoietic environment, have subsequently been examined as a component of this disrupted architecture. These plastic-adherent ex vivo–expanded cells expressing defined stromal markers display transcriptional abnormalities, altered differentiation characteristics, and a reduced ability to support HSPCs in MDS (see Raaijmakers9 and Li and Calvi10 for additional details), suggesting a potential role in BM failure in MDS. Critical contributions of the BM microenvironment have further been suggested by the inability to propagate human MDS cells in a cell-autonomous manner.11 Finally, the intriguing phenomenon of donor cell–derived hematopoietic neoplasm, defined as oncogenic transformation of apparently normal transplanted donor cells in the allogeneic patient environment, but not in the donor,12 suggests a potential contribution of ancillary cells to disease initiation of MDS. Albeit infrequent, these cases seem to defy the dogma that events initiating MDS and its defining characteristics are completely hematopoietic cell autonomous. Some investigators have suggested that the curative potential of allogeneic HSPC transplantation argues against pivotal contributions of the BM niche to disease initiation and progression. Cure, however, remains infrequent; in these patients, a necessary (but not sufficient) contribution of the BM microenvironment to disease pathogenesis, in cooperation with genetically aberrant HSPCs, cannot be excluded. Collectively, these observations and considerations challenge the view that ineffective hematopoiesis and leukemic transformation are exclusively driven by hematopoietic cell–autonomous events in human MDS and formed the rationale for experimental approaches to better define a potentially causative or permissive role of the microenvironment in human MDS in the last decade.
Experimental definition of niche contributions to MDS
The identification of functional components of the HSC niche at cellular resolution in mice13,14 has enabled experiments addressing their contributions to hematopoietic disease. Experimental support for a potential pivotal contribution of the BM niche to the pathogenesis of MDS was first provided in a study showing that dysfunction of a well-defined stromal subset of bone progenitor cells can initiate myelodysplasia and leukemia predisposition.15 Targeted deletion of the microRNA-processing endonuclease Dicer1 from osterix-expressing osteoprogenitor cells, but not mature osteoblasts, recapitulated key features of human MDS. This included the propensity to develop, at low frequency, AML harboring distinct cytogenetic abnormalities. Dicer1-deleted osteoprogenitor cells expressed reduced levels of Sbds, the gene mutated in Shwachman-Diamond syndrome (SDS), a human congenital BM failure and leukemia-predisposition disorder. Deletion of Sbds from osteoprogenitor cells phenocopied myelodysplasia in mice. The data demonstrated the central role that individual cellular elements of “stroma” can play in tissue homeostasis and revealed that primary dysfunction of such cells can initiate secondary neoplastic disease in the hematopoietic system. The work postulated a “niche-based” model of oncogenesis whereby an altered microenvironment cell can initiate a multistep process toward malignancy in hematopoietic cells.
The work furthered earlier studies in mice pointing at a potential driving impact of external signaling in the initiation of hematopoietic disease. Deletion of specific genes from the microenvironment, including RARγ and the inhibitor of NF-κB (IkBa), resulted in myeloproliferative neoplasms (MPNs)16 and a chronic myelomonocytic leukemia–like disease17 via Notch1 activation in hematopoietic cells, respectively
More recently, murine studies have more specifically implicated mesenchymal cells in the pathogenesis of human MDS and related disorders. An activating mutation of β-catenin in mesenchymal cells resulted in the development of myelodysplastic alterations and AML with common genetic aberrations.18 Activated β-catenin stimulates expression of jagged1 in mesenchymal cells, with subsequent activation of Notch signaling in HSPCs driving leukemic evolution. Human disease relevance was suggested by the increased β-catenin signaling and nuclear accumulation in osteolineage cells in a subset of MDS patients. Genetic or pharmacological inhibition of Notch signaling ameliorates AML and demonstrates the pathogenic role of the Notch pathway.
Mutations in Ptpn11 in mesenchymal precursor cells (MPCs), in the context of the congenital Noonan syndrome, promoted a juvenile myelomonocytic leukemia (JMML)-like disease and caused donor cell–derived MPN following stem cell transplantation.19 Similarly, deficiency of Sipa1 in the microenvironment is sufficient to drive an MDS/MPN-like phenotype in mice, with resemblance to the Dicer1 model, including reduction of B-cell progenitors, impaired osteogenic potential of MPCs, and the occurrence of myelodysplastic features in hematopoietic cells.20
Relevance of mesenchymal contributions to human MDS was further confirmed in experiments showing that patient-derived BMDSCs display a disturbed differentiation program and are essential for the propagation of MDS-initiating Lin−CD34+CD38− stem cells in xenograft models.21 Coinjection of CD34+ hematopoietic cells obtained from lower-risk (LR) MDS patients, together with their corresponding in vitro–expanded mesenchymal stromal cells, into the marrow cavity of NOD/LtSz-scid-IL2rg−/− mice significantly increases reconstitution with human MDS cells in comparison with transplantation with CD34+ alone or coinjection with BMDSCs from age-matched normal BM.
Finally, in vivo data in a transgenic murine model of NUP98-HOXD13 (NHD13) driven MDS have supported the relevance of the BM microenvironment in disease pathogenesis, because transplantation of NHD13 hematopoietic cells into wild-type recipients compared with NHD13 recipients resulted in a lower rate of leukemic progression and death, as well as improved hematopoiesis.22
Taken together, the data provide strong support for the conceptual view of “niche-driven malignant transformation,” in which primary alterations in niche cells drive the dysfunction and malignant transformation of supported, but distinct, HSPCs. Vice versa, primary (epi)genetic alterations in hematopoietic cells have the ability to alter mesenchymal niche components, such that niche cells facilitate disease propagation, supporting a concept of “niche-facilitated malignant transformation.”23-27 Importantly, the molecular mechanisms driving these biologic concepts may be overlapping, as discussed in the next section.
What are the molecular mechanisms driving niche contributions to MDS pathobiology?
The mouse experiments providing proof of principle of niche contributions to MDS pathogenesis have generated important insights into the underlying molecular mechanisms. Here, we will discuss the pathways that have emerged as key drivers of the MDS phenotype, supported by in vivo data and complemented by observations in human MDS.
Activation of inflammatory programs in MPCs: insights from congenital leukemia predisposition syndromes
Congenital monogenic BM failure syndromes with leukemia predisposition have provided unprecedented models to interrogate the contribution of the niche to MDS and leukemic evolution.
TP53-s100A8/A9-Toll-like receptor 4 signaling
SDS is caused by constitutive biallelic loss-of-function mutations in the ribosome biogenesis gene SBDS. Targeted deletion of Sbds, specifically in MPCs, faithfully recapitulates human disease.28 The Sbds-deficient environment induced mitochondrial dysfunction, oxidative stress (induction of reactive oxygen species [ROS]), and DNA damage in HSPCs. This was achieved via secretion of an array of inflammatory ligands, including the alarmins S100A8 and S100A9, downstream of Tp53 activation, ultimately resulting in Tp53-activation–associated apoptosis in HSCs, in part via TLR4 signaling. Transcriptional activation of this signaling axis in the mesenchymal niche was found in a subset of LR MDS patients, and it predicted leukemic evolution and progression-free survival. The data support the idea that inflammatory alterations in the mesenchymal niche attenuate the functional integrity of HSCs and may facilitate genetic alterations and subsequent transformation.
NF-κB–chemokine (CCL3)–IL-1β signaling
The notion that inflammatory alterations in mesenchymal niche cells may drive leukemic evolution was further supported by a study demonstrating a pivotal contribution of the mesenchymal niche to the genesis of JMML in Noonan syndrome.19 Mechanistically, inflammatory alterations, including Ccl3 upregulation in mesenchymal cells, recruited cells of the myeloid lineage to putative HSC niches. Subsequently, HSCs are “hyperactivated” by interleukin-1β (IL-1β) and possibly other proinflammatory cytokines. It appeared that persistently high levels of proinflammatory cytokines produced by the recruited monocytes displaced HSCs from MPC niches that are essential for maintaining HSC dormancy, resulting in their activation and, ultimately, donor cell–derived MPN in a transplantation-based mouse model. Administration of Ccl3 receptor antagonists effectively reverses the development of JMML-like disease induced by the Ptpn11-mutated BM microenvironment.
Overexpression of Ccl3 and other inflammatory factors in mesenchymal niche cells has been reported in a variety of mouse models of myeloid malignancies,22-24 including the SDS niche model,28 as well as mesenchymal cells of MDS patients.29,30 Highly purified CD271+ primary mesenchymal elements from LR MDS patients display global activation of inflammatory programs with a remarkable abundance of signatures related to NF-κB, EGF, tumor growth factor-β, and tumor necrosis factor signaling.29 Inflammatory alterations include upregulation of CCL3, TGFβ, IL6, and IL8 and a wide variety of factors previously demonstrated to be negative regulators of hematopoiesis, in particular erythropoiesis and B lymphopoiesis, differentiation programs typically affected in LR MDS. It is conceivable that the activin receptor type II ligand traps, such as sotatercept, exert part of their clinical effect through modulation of such inhibitory stromal factors.31 Mesenchymal NF-κB activation is a driver of the transcriptional upregulation of these inflammatory programs in LR MDS patients and results in ex vivo attenuation of HSPC numbers and function.32 These findings in human MDS complement murine studies implicating NF-κB activation in ancillary cells as a driver of “MDS/MPN-like” disease17,33 and may be seen in the context of NF-κB–driven chronic tissue inflammation as a driver of cancer initiation and progression via secretion of cytokines and soluble factors in other solid cancer models.34,35
Together, the data indicate that inflammation, specifically driven by MPCs in the hematopoietic niche directly or indirectly, leads to attenuation of HSC homeostasis and facilitates neoplastic transformation of the BM.
Inflammation in the BM in MDS: location may matter
The HSPC niche is an anatomically defined regulatory environment that governs the behavior of HSPCs. It is important to note that, although inflammatory alterations in the BM in MDS likely involve many cell types (including innate and adaptive immune cells), the proximity of such an inflammatory cell to (leukemia-initiating) HSPCs may be pivotal in its contribution to malignant transformation. Inflammatory ligands, coming from niche cells and other hematopoietic subsets present in the BM, may exert their biological effects specifically in the vicinity of the producing cell. For example, the local accumulation of S100A8/A9 in the environment is very high (up to 100 mg/mL and ∼50- to 100-fold higher than systemic concentrations), likely caused by attachment to extracellular matrices, such as proteoglycans.36 This implies that the exposure of HSPCs to S100A8/A9 is projected to relate strongly to their anatomical proximity to a producing cell. CD271+ mesenchymal cells are directly adjacent to CD34+ HSPCs in human BM.37 This notion of “spatial relevance” of inflammatory alterations in the BM may also be congruent with recent observations that aberrant overexpression of S100A8/A9 in hematopoietic/erythroid cells within the erythroid island niche in a model of human del(5q) MDS leads to a predominant erythroid anemic phenotype.38,39 The combined data suggest that similar inflammatory axes may determine distinct aspects of the MDS phenotype related to distinct niches.
Activation of Wnt signaling in mesenchymal cells
The finding that an activating mutation in β-catenin in mouse osteolineage cells can be the initiating event in MDS/AML18 should be interpreted in the context of a number of other studies pointing at a potential role for activation of β-catenin signaling in the mesenchymal environment in the pathogenesis of MDS.
Mice with haploinsufficiency for Apc develop an MDS-like phenotype that is characterized by macrocytic anemia and monocytosis.40-42 Again, MDS is driven by the microenvironment in these mice, as demonstrated by the finding that transplantation of normal hematopoietic cells into lethally irradiated Apcdel/+ recipients resulted in a fatal macrocytic anemia. Transplantation of Tp53+/− donor cells into Apcdel/+ mice resulted in the development of an immature lymphoid leukemia, suggesting that the microenvironment is conducive to developing neoplastic disease.43 Loss of 1 copy of Ctnnb1 was sufficient to prevent or delay the development of MDS in these mice, providing more formal genetic proof that altered canonical Wnt signaling in the microenvironment was responsible for the disease.42 Pyrvinium, a US Food and Drug Administration–approved drug, dampened Wnt signaling in mesenchymal niche cells isolated from this mouse model and patients with del(5q) MDS and delayed/inhibited disease in Apcdel/+ mice, even when it was administered after the presentation of anemia.
Activated WNT signaling in mesenchymal cells in MDS may result from epigenetic alterations induced by aberrant hematopoietic cells, as suggested by a study interrogating the DNA methylome of BMDSCs from MDS patients.44 This revealed widespread aberrant cytosine hypermethylation, including the WNT pathway antagonist FRZB, which was underexpressed in MDS-derived mesenchymal cells. Hypermethylation of cytosine guanine dinucleotides in the FRZB promoter could be induced in mesenchymal cells by coculture with leukemic cells (KG1A) and led to activation of WNT in HSPCs. A WNT/β-catenin–activated signature was also found in CD34+ cells from advanced cases of MDS, associated with adverse prognosis. Constitutive activation of β-catenin in hematopoietic cells yielded lethal myeloid disease in a NUP98-HOXD13 mouse model of MDS, confirming its role in disease progression. Interestingly, the hypomethylating agent 5-azacitidine may exert part of its clinical effect through modulation of WNT signaling in mesenchymal cells.44
Taken together, the data point to activation of WNT signaling in mesenchymal cells as a driver of BM failure and malignant transformation in MDS. Downstream signaling pathways remain largely undetermined, but they may include heterotypic NOTCH and WNT activation in HSPCs, targets for pharmacologic modulation, at least in the experimental setting of mouse modeling.
Loss of mesenchymal support for normal hematopoiesis
Neoplastic HSPCs may damage the HSC niche, undermining the support for residual normal HSCs and, through this mechanism, promote a competitive advantage. Limited data suggest that the frequency of mesenchymal cells is not reduced in MDS.29,45 However, in many studies, BMDSCs from MDS patients display reduced proliferative potential, cellular senescence, and reduced hematopoietic support.29,46-48 Also, reduced expression of HSC maintenance factors, such as CXCL12, has been reported in mesenchymal cells in mouse models19,22 and human disease.29 Although cellular senescence in mesenchymal cells awaits confirmation in nonexpanded mesenchymal cells in MDS, the potential relevance is stressed by recent findings in mouse models of myeloid neoplasm. In MPN, sympathetic nerve fibers supporting mesenchymal stem cells are consistently reduced in patients and mice expressing the JAK2(V617F) mutation in HSCs.24 MSC reduction is caused by BM neural damage and Schwann cell death triggered by IL-1β produced by mutant HSCs. AML leads to remodeling of vasculature caused by the production of proinflammatory and antiangiogenic cytokines that gradually degrades endosteal endothelium, mesenchymal cells, and osteoblasts.25 This damage to the endothelial and mesenchymal niches results in reduced support for residual normal hematopoiesis and progression to leukemia. Similarly, in xenograft models of AML, disruption of the adipogenic niche resulted in impaired erythropoiesis and myelopoiesis.26 Finally, it was shown in Flt3-ITD mice that Flt3-ITD–expressing cells induced a reduction in mesenchymal and endothelial cells with increased inflammation-associated gene expression, resulting in disease progression at the expense of normal hematopoiesis.27
Importantly, these proposed molecular mechanisms underlying niche-facilitated BM failure and leukemic evolution may not be mutually exclusive. WNT/β-catenin signaling can be upregulated in inflammatory processes.49 NF-κB, through inhibiting glycogen synthase kinase-3β, activates the canonical WNT pathway.49 Paracrine inflammatory signaling converging on the NF-κB pathway has further been linked to the induction of cellular senescence.50-52 In addition, activation of NF-κB in mesenchymal cells results in cellular stress and impaired proliferation of mesenchymal cells.32 Similarly, tumor growth factor-β signaling is induced in primary mesenchymal cells from MDS patients and may induce cellular senescence in BMDSCs.53 Cellular senescence, in turn, may develop a chronic prosecretory state, termed the senescence-associated secretory phenotype,54 comprising a plethora of inflammatory ligands that play a major stimulatory role in cancer progression.
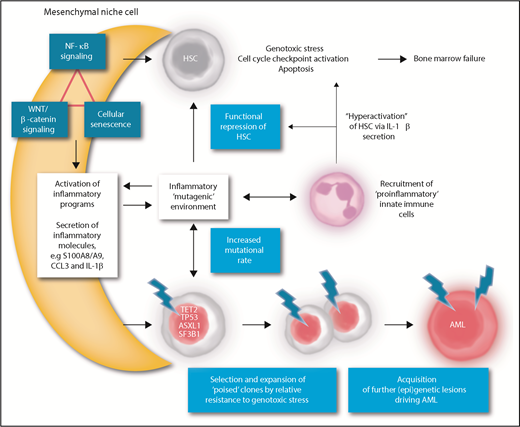
Collectively, the data suggest that an intricate network of inflammation, NF-κB, and WNT/β-catenin activation and cellular senescence may create a local environment promoting HSC dysfunction and leukemic transformation in MDS (Figure 1).
A model of mesenchymal niche–facilitated clonal evolution in MDS. NF-κB activation, β-catenin/WNT activation, and/or senescence-associated signaling may induce inflammatory alterations in mesenchymal niche cells that result in secretion of inflammatory ligands, such as S100A8/A9, C-C chemokines, and IL-1β in the HSPC niche. Mesenchymal inflammation may be the result of primary (genetic or epigenetic) alterations in mesenchymal cells or be induced by inflammatory alterations in clonal hematopoietic cells “starting the fire.” The local inflammatory niche induces functional repression of HSCs through direct receptor-mediated genotoxic stress and/or recruitment of proinflammatory innate immune cells, further providing an inflammatory feed-forward loop. The inflammatory stem cell niche may drive the accelerated emergence of mutations in HSPCs through direct genotoxic signaling or the induction of replicative stress. Cells harboring “poising” mutations (ie, mutations that confer resistance to inflammation-induced apoptosis) are selected for in the inflammatory environment by virtue of their relative resistance to inflammation-associated genotoxic stress. The presence of a poising mutation, in an inflammatory environment, accelerates the acquisition of additional genetic events and transformation to frank AML. Thus, inhibition of inflammatory signaling, targeting ligands such as S100A8/A9, C-C chemokines, and IL-1β signaling or related receptors, is anticipated to alleviate the genotoxic stress on normal HSPCs, relieve the selective pressure on preleukemic clones, and attenuate clonal evolution and leukemic progression in MDS.
A model of mesenchymal niche–facilitated clonal evolution in MDS. NF-κB activation, β-catenin/WNT activation, and/or senescence-associated signaling may induce inflammatory alterations in mesenchymal niche cells that result in secretion of inflammatory ligands, such as S100A8/A9, C-C chemokines, and IL-1β in the HSPC niche. Mesenchymal inflammation may be the result of primary (genetic or epigenetic) alterations in mesenchymal cells or be induced by inflammatory alterations in clonal hematopoietic cells “starting the fire.” The local inflammatory niche induces functional repression of HSCs through direct receptor-mediated genotoxic stress and/or recruitment of proinflammatory innate immune cells, further providing an inflammatory feed-forward loop. The inflammatory stem cell niche may drive the accelerated emergence of mutations in HSPCs through direct genotoxic signaling or the induction of replicative stress. Cells harboring “poising” mutations (ie, mutations that confer resistance to inflammation-induced apoptosis) are selected for in the inflammatory environment by virtue of their relative resistance to inflammation-associated genotoxic stress. The presence of a poising mutation, in an inflammatory environment, accelerates the acquisition of additional genetic events and transformation to frank AML. Thus, inhibition of inflammatory signaling, targeting ligands such as S100A8/A9, C-C chemokines, and IL-1β signaling or related receptors, is anticipated to alleviate the genotoxic stress on normal HSPCs, relieve the selective pressure on preleukemic clones, and attenuate clonal evolution and leukemic progression in MDS.
Niche abnormalities in MDS: chicken or egg?
Although it is has become evident that molecular alterations in mesenchymal cells in MDS exist and contribute to disease pathogenesis, it remains unclear whether those alterations are cell intrinsic or induced by aberrant hematopoietic cells. The answer to this question is likely to be context dependent. In the congenital BM failure and leukemia-predisposition syndromes, the primary event in niche cells is the germline genetic abnormality defining the disorder. Mouse models have demonstrated that these genetic alterations in mesenchymal cells are sufficient to drive the hematopoietic phenotypes.19,20 In the case of acquired disorders, such as MDS, this “chicken or egg” question is more complicated to answer.
Evidence for genetic alterations in mesenchymal cells in MDS?
Mesenchymal cells in the BM are derived from a common progenitor cell that replenishes stromal elements with potentially differing functions.55 A mutation in a mesenchymal stem/progenitor cell may be propagated in its progeny, thus creating a niche field with aberrant biological behavior. This may evoke localized signaling in the niche, ultimately resulting in a local “mutagenic environment” driving malignant transformation or clonal selection of hematopoietic cells. Although conceptually attractive, this series of events would be very difficult to capture in situ in humans if restricted to a very small area of the BM.
Distinct cytogenetic abnormalities in BMDSCs from MDS patients have been reported,56-59 but the significance of these findings is uncertain. It is not always clear whether these alterations exist in situ or have been caused by serial ex vivo passaging of cells. Even if present in the MDS marrow, they may have originated from coevolution in a mutagenic environment rather than precede clonal evolution of hematopoietic cells. Finally, these cytogenetic aberrations have not been linked to aberrant signaling and downstream biological events relevant to the pathogenesis of MDS. Taken together, there seems to be insufficient experimental support for the view that somatic mutations or other genetic events in mesenchymal cells are the primary event in MDS and/or drive disease phenotypes.
However, it is conceivable that primary alterations in the mesenchymal niche, if they exist, are induced by exposure to environmental insults, such as toxic agents, including chemotherapy and irradiation.60-62 Moreover, aging is known to affect mesenchymal cell biology and elicit inflammatory responses from these cells,63,64 including alterations implicated in MDS pathology, such as activation of Wnt signaling and S100A8/A9 overexpression.65,66
Niche alteration as a consequence of genetic events in hematopoietic cells
Although primary (insult or aging-induced) genetic or epigenetic alterations may occur in mesenchymal cells in MDS, it seems more likely that primary genetic alterations in hematopoietic cells induce disruption of niche cells. Comparison of mesenchymal cells directly isolated from BM samples with their ex vivo–expanded counterparts (BMDSCs) indicated that many inflammatory programs are tissue context dependent,29 suggesting that interaction with other cells within the MDS BM is required to induce and maintain aberrant molecular programs. This view has been substantiated by coculture experiments in which healthy BMDSCs adopt aberrant MDS MSC-like molecular features when exposed to MDS hematopoietic cells, indicative of an instructive remodeling of the microenvironment.21 Those hematopoietic cell–induced changes may include epigenetic alterations,44 perhaps explaining why some features of MDS-derived BMDSCs are maintained through serial passaging. Future experiments would have to establish which hematopoietic cell subsets are involved in the cross talk with mesenchymal cells and whether this signaling is mutation specific. It is conceivable that inflammatory alterations in MDS HSPCs “start the fire” and subsequently induce inflammatory alterations in the mesenchymal niche. Indeed, MDS HSPCs manifest NLRP3 inflammasome activation39 (which may be a direct consequence of an oncogenic mutation, as has been demonstrated for TET267 ) that directs the generation of IL-1β, S100A9, and ROS. S100A9-induced signaling, in turn, activates reduced NAD phosphate oxidase and increases levels of ROS that initiate β-catenin activation.39 Moreover, in a recent mouse model of del(5q) MDS, genetic alterations in hematopoietic cells were shown to result in an induction of S100A8/A9 expression in mesenchymal cells.68 Thus, one can envision a bidirectional amplifying inflammatory circuitry between mutated MDS cells and their mesenchymal niche driving and sustaining inflammation in MDS (Figure 1).
Niche-facilitated disease evolution in MDS: induction or selection of mutant clones?
Loss of quiescence, increased proliferative stress, and genotoxic stress or “hyperactivation” induced by alterations in the mesenchymal niche19,28 may poise HSPCs for increased DNA damage, promoting malignant transformation. As such, the niche may act as an initiator of oncogenesis. Alternatively, the inflammatory microenvironment in MDS may enable the expansion and, eventually, leukemic transformation, of preexisting genetic clones in the BM. Niche-derived inflammatory signaling may drive the functional repression of HSCs and the subsequent selection and clonal evolution of cells carrying a mutation, conferring resistance to this stress at the cost of “poising” the cell for malignant transformation (Figure 1). In this view, the microenvironment is a “facilitator” that is required, but not sufficient, for malignant transformation.
Clonal selection in the context of microenvironmental alterations has been documented in the context of ageing, toxic insults, and inflammation.69-71 Irradiation of mice promotes the selection of particular oncogenic mutations,72 including Tp5373 ; this is congruent with the fact that selection for TP53 mutations occurs during chemotherapeutic treatment and is relevant for the development of therapy-related AML.74 Similarly, ageing-associated chronic inflammation might be a driver of clonal selection,75 promoting leukemogenesis by providing an environment that is permissive to the expansion of “poised” preleukemic cells carrying founding mutations. For example, B-progenitor fitness reductions and increased selection for oncogene-bearing clones have been shown to be mediated by the inflammatory environment in aged mice.76 An inflammatory microenvironment may also induce the selection of TP53-mutated clones. Mutations in TP53 are present in the skin but provide a survival advantage only in response to chronic UV light exposure.77 Similarly, TP53 mutations in the intestine have a clonal advantage only in the setting of chronic inflammation,78 and clonal expansion of TP53 is observed in patients with irritable bowel disease79 but has not been recorded in the healthy colon mucosa. It is tempting to speculate that similar mechanisms may be at play in SDS or del(5q) MDS,80 in which leukemic transformation is often associated with TP53 mutations.43,81 Genotoxic stress in the SDS environment led to cell-cycle checkpoint activation and apoptosis in normal HSPCs.28 It is conceivable that mutations dampening the cell-cycle checkpoint may lead to competitive survival of mutated clones, perhaps at the expense of accelerated mutagenesis, a hypothesis that awaits experimental definition.
However, inflammation driving expansion of resistant clones, of relevance to human MDS, is beginning to be documented in the case of TET2 mutations. Tet2-knockout murine and TET2-mutant human HSPCs have a clonal advantage caused by resistance to apoptosis in an in vitro environment that contains the proinflammatory cytokine tumor necrosis factor-α (TNFα).82 TET2-deficient macrophages are hyperinflammatory,83 and this may exacerbate processes by propagating an inflammatory environment.67,84 Furthermore, increased IL-6 production or activation of TLR2 is critical for the development of the myeloproliferative phenotype of TET2-deficient hematopoietic cells.85
Together, the data point to necessary contributions of the aberrant (inflammatory) microenvironment to drive the induction, selection, and eventual expansion of mutant clones that are poised for subsequent malignant transformation (Figure 1). The data strongly argue that MDS needs to be considered a disease of a tissue rather than a disease of hematopoietic cells in isolation. Cross talk between HSPCs and their (mesenchymal) environment likely drives disease initiation and evolution. This has important consequences for our thinking about prognostication and treatment of this disease. Niche parameters should be considered complementing HSPC-intrinsic characteristics in future models of prognostication, and targeting environmental contributions (eg, by dampening inflammation through inhibition of TLR4 signaling, ROS,28 or the NLRP3 inflammasome39,67 ) may not only ameliorate BM failure but ultimately attenuate or prevent leukemic evolution if the selective pressure for poising clones can be alleviated.
Acknowledgment
This work was supported by Dutch Cancer Society (KWF Kankerbestrijding) grants EMCR 2016-10488 and 2017-11092.
Authorship
Contribution: E.P. wrote the article, provided literature background, and assisted in illustrating. M.H.G.P.R. wrote the article and supervised E.P.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc H. G. P. Raaijmakers, Erasmus MC Cancer Institute, Dr. Molewaterplein 50, 3015GE Rotterdam, The Netherlands; e-mail: m.h.g.raaijmakers@erasmusmc.nl.