Key Points
Rituximab added to ibrutinib in relapsed and treatment-naive high-risk patients with CLL failed to show improvement in progression-free survival.
Patients treated with ibrutinib plus rituximab reached their remissions faster and achieved significantly lower residual disease levels.
Abstract
Ibrutinib, an oral covalent inhibitor of Bruton’s tyrosine kinase, is an effective therapy for patients with chronic lymphocytic leukemia (CLL). To determine whether rituximab provides added benefit to ibrutinib, we conducted a randomized single-center trial of ibrutinib vs ibrutinib plus rituximab. Patients with CLL requiring therapy were randomized to receive 28-day cycles of once-daily ibrutinib 420 mg, either as a single agent (n = 104), or together with rituximab (375 mg/m2; n = 104), given weekly during cycle 1, then once per cycle until cycle 6. The primary end point was progression-free survival (PFS) in the intention-to-treat population. We enrolled 208 patients with CLL, 181 with relapsed CLL and 27 treatment-naive patients with high-risk disease (17p deletion or TP53 mutation). After a median follow-up of 36 months, the Kaplan-Meier estimates of PFS were 86% (95% confidence interval [CI], 76.6-91.9) for patients receiving ibrutinib, and 86.9% (95% CI, 77.3-92.6) for patients receiving ibrutinib plus rituximab. Similarly, response rates were the same in both arms (overall response rate, 92%). However, time to normalization of peripheral blood lymphocyte counts and time to complete remission were shorter, and residual disease levels in the bone marrow were lower, in patients receiving ibrutinib plus rituximab. We conclude that the addition of rituximab to ibrutinib in relapsed and treatment-naive high-risk patients with CLL failed to show improvement in PFS. However, patients treated with ibrutinib plus rituximab reached their remissions faster and achieved significantly lower residual disease levels. Given these results, ibrutinib as single-agent therapy remains current standard-of-care treatment in CLL. This trial was registered at www.clinicaltrials.gov as #NCT02007044.
Introduction
During the past few years, treatment of patients with chronic lymphocytic leukemia (CLL) underwent fundamental changes due to the introduction of new targeted therapies,1 such as kinase inhibitors targeting B-cell receptor (BCR) signaling,2-4 new monoclonal antibodies,5 and the BCL2 antagonist venetoclax.6,7 Ibrutinib (Ibr) is a potent, selective inhibitor of Bruton’s tyrosine kinase (BTK) that inactivates BTK through irreversible covalent bonding to Cys-481 in the adenosine triphosphate–binding domain of BTK.8 BTK, a member of the TEC family of kinases, becomes activated after BCR triggering by upstream signaling molecules, including spleen tyrosine kinase and phosphatidylinositol 3-kinases. Signaling downstream of BTK include activation phospholipase Cγ2, calcium mobilization, and transcriptional activation via NF-κB and extracellular signal–regulated kinase, resulting in B-cell survival and proliferation.9 BTK is also involved in the signaling and function of adhesion molecules (integrins)10 and chemokine receptors such as CXC-chemokine receptors 4 and 5.11 BTK inhibition consequently results in impaired CLL cell migration and adhesion,12,13 explaining the characteristic transient redistribution lymphocytosis due to mobilization of tissue-resident CLL cells into the peripheral blood and concurrent rapid normalization of the size of involved lymph nodes and spleen. Although the redistribution lymphocytosis eventually resolves in the vast majority of patients, most responses to single-agent Ibr in patients with CLL are partial remissions (PRs). Hence, combination therapies are currently explored in clinical trials to increase the rates of complete remission (CR) and minimal residual disease (MRD) negativity.14,15 Indeed, Ibr combination therapy with bendamustine and rituximab (BR),15 or venetoclax and the anti-CD20 monoclonal antibody obinutuzumab,16 can increase the rate of complete remissions, including remissions with undetectable MRD. However, whether these improvements in depth of remission translate into improvement in remission duration or survival has not been shown, and therefore Ibr monotherapy currently is considered standard of care. The addition of CD20 antibodies to chemotherapy as chemoimmunotherapy significantly improved the outcome of patients with CLL,5,17,18 and our previous experience with Ibr combined with rituximab (Ibr + R) showed high response rates and safety.14 We therefore conducted a randomized trial of Ibr vs Ibr + R to characterize the impact of adding rituximab to Ibr on progression-free survival (PFS) and overall survival (OS), depth of remission, and time to achieving remission.
Patients and methods
Patients
A total of 208 patients with CLL or small lymphocytic lymphoma were enrolled into a 2-arm, phase 2 study of Ibr vs Ibr + R at the MD Anderson Cancer Center between December 2013 and October 2017. Inclusion criteria included previously treated CLL/small lymphocytic lymphoma, with indication for treatment in accordance with the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria.19 Untreated patients with 17p deletion (del17p) or TP53 mutation were also permitted, given the poor outcome of these patients with standard frontline chemoimmunotherapy. Patients were required to have adequate renal and hepatic function, and absence of active infection. Patients with uncontrolled autoimmune hemolytic anemia or autoimmune thrombocytopenia, severe hematopoietic insufficiency, bleeding diathesis or coagulopathy, recent hemorrhagic events, or concomitant treatment with warfarin were excluded. Patients who received previous therapy with agents targeting BTK or other BCR pathway molecules (eg, idelalisib) were also excluded.
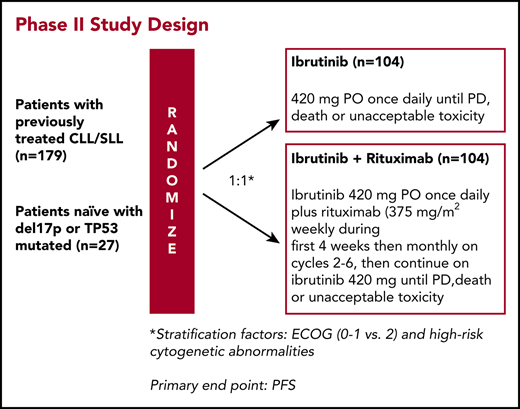
Study design
This phase 2 clinical trial was developed by the investigators in collaboration with Pharmacyclics LLC and approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki. Treatment consisted of Ibr (420 mg daily by mouth) continuously daily (1 cycle = 28 days), or Ibr in combination with weekly rituximab (Ibr + R, 375 mg/m2 intravenously) for weeks 1 to 4 (cycle 1); rituximab was then given once every 4 weeks until cycle 6, followed by single-agent Ibr. Patients remained on treatment until disease progression or toxicities precluded further therapy. Ibr was held for any grade 3/4 toxicity until the adverse event returned to baseline or resolved completely. If the grade 3/4 toxicity reoccurred, the dose of Ibr was reduced. Clinical and laboratory assessments were performed every week during cycle 1, then once every 4 weeks until cycle 6, every 3 cycles until cycle 24, and then every 6 cycles thereafter while patients remained in the study.
Response and safety assessments
Response assessment for PFS and overall response rate (ORR) included clinical assessment, along with radiologic examinations with computed tomography scans of the chest, abdomen, and pelvis at baseline, after 3 or 6 cycles, and after 12 cycles, and once every 12 cycles thereafter. Bone marrow aspirations and biopsies were performed and evaluated by using morphology and flow cytometry at baseline and after 12 and 24 cycles, and once every 12 cycles thereafter. MRD assessment of bone marrow samples was performed by using flow cytometry according to the international standardized methods of the European Research Initiative on CLL, with a sensitivity down to 0.01%.20 Responses were evaluated according to the 2008 IWCLL criteria,19 with the exception that lymphocytosis was not the sole criterion for disease progression. Patients with persistent lymphocytosis, who otherwise were considered to have a PR by all other measures, were considered to have a PR with lymphocytosis (PRL). To assess for disease progression on therapy, best response (ie, the true nadir) was considered as reference. For example, if a patient had normalization or major reduction of absolute lymphocyte counts (ALC) on therapy but later developed progressive lymphocytosis, this patient would be considered to have progressive disease, especially in the context of other signs (clinical and laboratory) of disease progression, such as progressive cytopenias, increasing levels of lactate dehydrogenase, and/or organomegaly and/or progressive lymphadenopathy. Safety monitoring was performed weekly for the first cycle, then every 4 weeks until cycle 6, every 3 cycles until cycle 24, and then every 6 cycles thereafter while patients remained in the study. Adverse events were graded by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
In this single-center, phase 2 randomized clinical trial, patients with CLL were randomized in a 1:1 ratio to receive single-agent Ibr or Ibr + R. The primary objective was to compare the PFS rate. Secondary objectives were to determine the safety and tolerability, the ORR, the estimated PFS in patients with CLL, and biomarker responses in patients with CLL receiving Ibr vs Ibr + R. Patients in CR, partial remission (PR), or stable disease were all counted as progression-free. PFS time was defined as the number of months from treatment date to progression or death date. Patients were censored for PFS at the last clinical assessment before receipt of new antileukemia therapy, or after loss to follow-up, whichever occurred first.
To ensure patient characteristics were balanced across arms, patients were stratified on the basis of CLL cytogenetic risk factors (TP53 mutation/del17p, del11q without del17p or TP53 mutation, or none/unknown) and the Eastern Cooperative Oncology group performance status. A total sample size of 208 patients (104 patients in each arm) was chosen to have a 81.3% power to detect a 15% difference reflected in the PFS rates at 2 years with a 2-sided significance level of 0.05, assuming that the 2-year PFS for Ibr monotherapy is 74%21 and the proposed 2-year PFS rate for the combination treatment is 89%. The Department of Biostatistics provided and maintained a Web site (“Clinical Trial Conduct,” https://biostatistics.mdanderson.org/ClinicalTrialConduct/) for enrolling patients in this study and evaluating the efficacy monitoring rules described earlier. This analysis presents the final data from our study, extracted on January 3, 2018. PFS was analyzed by using the Kaplan-Meier method. The stratified log-rank test was used as the primary analysis for PFS, and subgroup analyses were performed based on an unstratified Cox model. OS was analyzed similarly. The intention-to-treat population included all randomized patients, and the safety population included patients who received at least 1 dose of study drug.
Statistical analyses were conducted by using STATA/SE version 12.1 (Stata LP, College Station, TX) and GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA; 2013). This study was registered at www.clinicaltrials.gov as #NCT02007044.
Results
Patients and treatment
Patients were accrued between December 2013 and October 2017 at MD Anderson Cancer Center. Baseline patient characteristics are summarized in Table 1. Demographic and clinical characteristics were balanced between the 2 treatment arms. Patients’ median age was 65 years (range, 42-83 years), and most patients had 1 to 2 lines of previous therapy (Ibr, 65%; Ibr + R, 73%). The median number of previous therapies for patients taking Ibr was 1 (range, 0-7) and for patients receiving Ibr + R it was also 1 (range, 0-10). Twenty-seven patients were untreated (all with del17p and/or TP53 mutation; Ibr, 14%; Ibr + R, 12%). A total of 56 patients had del17p (Ibr, 25%; Ibr + R, 29%), 50 patients had TP53 mutation (Ibr, 28%; Ibr + R, 20%), 77 patients had del17p and TP53 mutation (Ibr, 39.4%; Ibr + R, 34.6%), and 42 patients had 11q deletion (Ibr, 26%; Ibr + R, 14%). A total of 123 patients had unmutated IGHV genes (Ibr, 59%; Ibr + R, 60%).
Patient characteristics
| Characteristic . | Ibr (n = 104) . | Ibr + R (n = 104) . |
|---|---|---|
| Sex | ||
| Male | 75 (72.1) | 71 (68) |
| Age, y | 65 (44-83) | 65 (42-81) |
| ECOG performance status | ||
| 0 | 3 (3) | 4 (4) |
| 1 | 101 (97) | 100 (96) |
| Rai stage | ||
| III-IV | 38 (37) | 42 (40) |
| Lines of prior treatment | ||
| 0 | 15 (14) | 12 (12) |
| 1-2 | 68 (65) | 76 (73) |
| 3 | 10 (10) | 7 (7) |
| ≥4 | 11 (11) | 9 (9) |
| IGHV status | ||
| Unmutated | 61 (59) | 62 (60) |
| Cytogenetics, FISH | ||
| 11q deletion | 27 (26) | 15 (14) |
| del17p | 26 (25) | 30 (29) |
| TP53 status | ||
| Mutated | 29 (28) | 21 (20) |
| ZAP-70 | ||
| Positive | 65 (63) | 63 (61) |
| CD38 | ||
| Positive | 52 (50) | 50 (48) |
| β2-macroglobulin, mg/L | 3.7 (1.3-13.1) | 3.7 (1.4-11.9) |
| White blood cell count, K/μL | 36.7 (2.8-361.8) | 36.8 (1.2-321.4) |
| Hemoglobin, g/dL | 12.6 (8.0-17.0) | 12.4 (7.3-16.3) |
| Platelets, K/μL | 122 (30-368) | 127 (24-465) |
| Characteristic . | Ibr (n = 104) . | Ibr + R (n = 104) . |
|---|---|---|
| Sex | ||
| Male | 75 (72.1) | 71 (68) |
| Age, y | 65 (44-83) | 65 (42-81) |
| ECOG performance status | ||
| 0 | 3 (3) | 4 (4) |
| 1 | 101 (97) | 100 (96) |
| Rai stage | ||
| III-IV | 38 (37) | 42 (40) |
| Lines of prior treatment | ||
| 0 | 15 (14) | 12 (12) |
| 1-2 | 68 (65) | 76 (73) |
| 3 | 10 (10) | 7 (7) |
| ≥4 | 11 (11) | 9 (9) |
| IGHV status | ||
| Unmutated | 61 (59) | 62 (60) |
| Cytogenetics, FISH | ||
| 11q deletion | 27 (26) | 15 (14) |
| del17p | 26 (25) | 30 (29) |
| TP53 status | ||
| Mutated | 29 (28) | 21 (20) |
| ZAP-70 | ||
| Positive | 65 (63) | 63 (61) |
| CD38 | ||
| Positive | 52 (50) | 50 (48) |
| β2-macroglobulin, mg/L | 3.7 (1.3-13.1) | 3.7 (1.4-11.9) |
| White blood cell count, K/μL | 36.7 (2.8-361.8) | 36.8 (1.2-321.4) |
| Hemoglobin, g/dL | 12.6 (8.0-17.0) | 12.4 (7.3-16.3) |
| Platelets, K/μL | 122 (30-368) | 127 (24-465) |
Data are expressed as no. (%) or median (range). ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; ZAP-70, zeta-chain-associated protein kinase 70.
After a median follow-up of 36 months (Ibr, 35.8 months [range, 3.0-47.1 months]; Ibr + R, 36.4 months [range, 2.9-47.8 months]), 138 (66%) patients continued on therapy and 70 (34%) discontinued treatment. Among the patients who discontinued, 6 patients died while enrolled in the study. Thirty patients (14%) discontinued therapy because of toxicity, such as infection (n = 8), atrial fibrillation (n = 6), diarrhea and nausea (n = 3), arthralgia (n = 3), skin rash (n = 2), bleeding events (n = 2), autoimmune-mediated cytopenias (n = 2), and other toxicities. Thirteen patients (6%) stopped due to disease progression and 21 for other reasons, such as treatment of other cancers (n = 7 [ie, 2 cases of chronic myeloid leukemia and 1 case each of colorectal cancer, melanoma, liposarcoma, recurrent squamous cell cancer, and bladder cancer]), noncompliance, or to pursue other CLL treatment. Analyses of annual discontinuation rates revealed that the number of patients in both arms who discontinued treatment was greater within the first year of therapy, where the annual discontinuation rates were 15.7% and 21.3% for patients receiving Ibr or Ibr + R, respectively, and then declined to 9.1% and 11.4% during the second and third years for patients receiving Ibr and to 17.1% and 6.6% for patients receiving Ibr + R (supplemental Table 19, available on the Blood Web site).
PFS and treatment responses
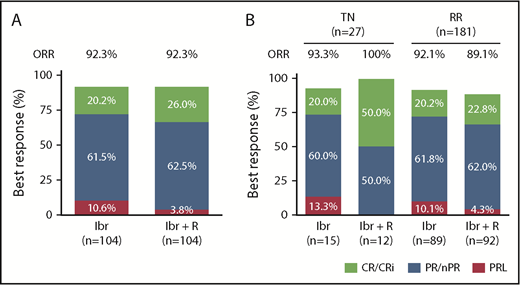
A total of 208 patients were enrolled and evaluated for responses per the 2008 IWCLL guidelines. Among the patients receiving Ibr alone, 21 (20.2%) achieved a complete remission (CR), 64 (61.5%) achieved a partial remission (PR), and 11 (10.6%) achieved PRL as best response (Figure 1). Among the patients treated with Ibr + R, 27 (26%) achieved a CR, 65 (62.5%) a PR, and 4 (3.8%) a PRL, accounting for an ORR of 92.3%. The higher CR rate in patients receiving Ibr + R was not significant (P = .32) (supplemental Table 2). This trend for higher CR rates in patients receiving Ibr + R combination therapy was more noticeable in patients with del17p and/or TP53 mutation (Ibr, 22% CR; Ibr + R, 33% CR) and previously untreated CLL (Ibr, 20% CR; Ibr + R, 50% CR). Interestingly, one-half of the CRs in patients with previously untreated CLL who received Ibr + R were MRD-negative in the bone marrow. However, the sample size in these subgroups was small. In all disease subsets, responses were similarly distributed, with a majority of patients achieving a PR/PRL, and a substantial number of patients achieving a CR. The responses in different subgroups of patients are detailed in supplemental Table 3. Response assessment at different time points (12 and 24 months) revealed that patients receiving Ibr + R achieved 17.3% CR, and 68.3% achieved PR/nodular PR (nPR) after 12 months, compared with 5.8% CR and 68.3% PR/nPR in patients receiving Ibr alone. After 24 months of Ibr + R, 25% of patients achieved a CR and 63.5% a PR/nPR, compared with 18.3% CR and 63.5% PR/nPR in patients receiving Ibr alone (supplemental Table 21).
Best responses to treatment with Ibr or Ibr + R in CLL. (A) Responses in patients with CLL treated with Ibr or Ibr + R. CRs, including CRs with incomplete count recovery (CR/Cri), are depicted in green; PRs, including PR/nPRs, are in blue; and PRLs are in pink. Although the CR rate with the addition of rituximab was higher, it did not reach statistical significance. (B) Responses in treatment-naive (TN) and relapsed/refractory (RR) patients with CLL treated with Ibr or with Ibr + R.
Best responses to treatment with Ibr or Ibr + R in CLL. (A) Responses in patients with CLL treated with Ibr or Ibr + R. CRs, including CRs with incomplete count recovery (CR/Cri), are depicted in green; PRs, including PR/nPRs, are in blue; and PRLs are in pink. Although the CR rate with the addition of rituximab was higher, it did not reach statistical significance. (B) Responses in treatment-naive (TN) and relapsed/refractory (RR) patients with CLL treated with Ibr or with Ibr + R.
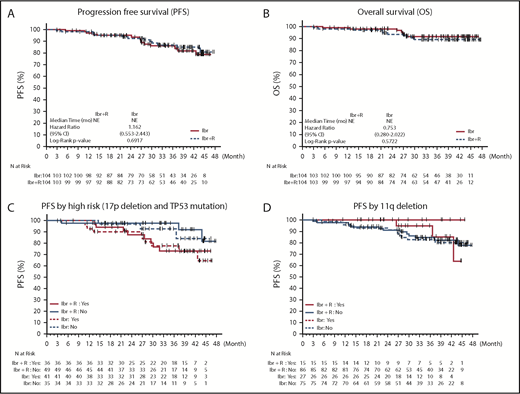
The PFS rates at 2 years were 95% (95% confidence interval [CI], 88.4-97.9) for patients receiving Ibr alone and 92.5% (95% CI, 84.9-96.4) for patients receiving Ibr + R (Figure 2A). At 36 months, an estimated 86% (95% CI, 76.6-91.9) of patients receiving Ibr alone and 86.9% (95% CI, 77.3-92.6) of patients receiving Ibr + R were alive and free of progression (hazard ratio, 1.04 [95% CI, 0.49-2.20]; P = .912) (supplemental Table 4). The median PFS was not reached in either treatment arm. In patients with del17p or TP53 mutation, the estimated 36-month PFS rate was lower in both arms: 77.7% (95% CI, 60-88.3; n = 41) in patients receiving Ibr and 73.1% (95% CI, 53-85.6; n = 36) in patients receiving Ibr + R (Figure 2C; supplemental Table 5). In patients with 11q deletion, the estimated PFS after 36 months was 94.7% (95% CI, 68.1-99.2; n = 27) in patients receiving Ibr and 100% (n = 15) in patients receiving Ibr + R (Figure 2D; supplemental Table 6).
Kaplan-Meier curves for PFS and OS. The top panels show the probability of PFS (A) and OS (B) and for all 208 patients. The bottom panels depict PFS with respect to del17p status (C) and 11q deletion status (D). Tick marks indicate censored data.
Kaplan-Meier curves for PFS and OS. The top panels show the probability of PFS (A) and OS (B) and for all 208 patients. The bottom panels depict PFS with respect to del17p status (C) and 11q deletion status (D). Tick marks indicate censored data.
Fifteen patients had disease progression, 10 receiving Ibr alone and 5 receiving Ibr + R; among these patients, 10 had CLL progression and 5 Richter transformation (4 cases of diffuse large B-cell lymphoma and 1 case of plasmablastic lymphoma). Median time to disease progression was 26 months (range, 10-43 months). Three patients with disease progression died of their underlying disease or of respiratory failure. Event-free survival, defined as the time from study entry to disease progression, re-treatment, or death due to any cause, was similar in both treatment arms. For example, the estimated 24-month event-free survival was 95% (95% CI, 88.4-97.9) in patients receiving Ibr and 92.5% (95% CI, 84.9-96.4) in patients receiving Ibr + R (supplemental Figure 1; supplemental Table 20). The median event-free survival was not reached in either treatment arm.
Overall survival
The estimated OS at 36 months was 92% (95% CI, 83.5-96) in patients receiving Ibr alone and 89% (95% CI, 80.1-94.3) in patients receiving Ibr + R (Figure 2B; supplemental Table 7). Seven patients receiving Ibr alone and 9 patients receiving Ibr + R died. Among the 6 patients who died while enrolled, 1 patient had multiorgan failure (bowel obstruction, kidney failure) and suspected Richter transformation after 3 months of study, 1 patient died of a central nervous system hemorrhage after 27 months of therapy, 1 patient died of colon perforation following an embolization of a colon hematoma after 5 months of study, 1 patient died of pneumonia after 14 months of study, 1 died of unknown causes (during sleep) after 27 months of study, and 1 died of respiratory failure and disease progression after 13 months of study. The 10 other patients who died after study discontinuation died of infectious complications (n = 4), disease progression (n = 2), other cancers (n = 2 [ie, metastatic colon cancer and melanoma]), or of unknown causes (n = 2).
Time to response and MRD
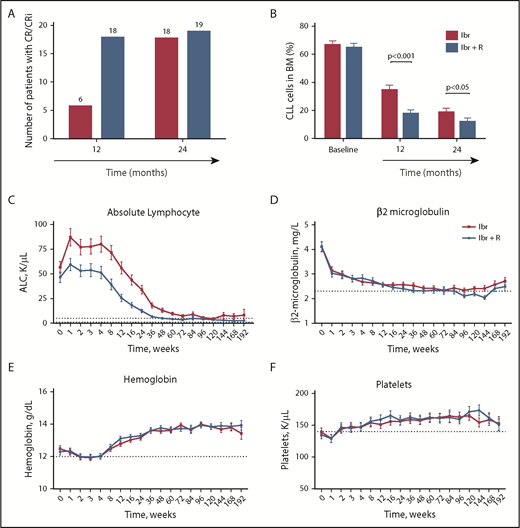
Time to response, including any type of response (ie, time from start of therapy to initial date of CR, PR, or PRL), was similar in patients receiving Ibr (median, 4.7 months; range, 4.2-11.8 months; n = 96) or Ibr + R (median, 4.8 months; range, 1.9-11.3 months; n = 96) (supplemental Table 8). However, the median time to achieving a CR was shorter in patients receiving Ibr + R (11.5 months; range, 4.8-35.5 months; n = 27) than in patients taking Ibr alone (22.2 months; range, 10.5-44.7 months; n = 21); times to CR in each disease subgroup are summarized in supplemental Table 9. Consequently, more patients receiving Ibr + R were in CR at 12 months than those receiving Ibr alone; this difference diminished at 24 months (Figure 3A). Serial bone marrow evaluations for MRD according to flow cytometry revealed significantly lower levels of MRD in patients receiving Ibr + R (Table 2; Figure 3B). The mean bone marrow MRD level after 12 months of therapy with Ibr alone was 34.4% (n = 89; 95% CI, 29.9-39.0) and 18.5% in patients receiving Ibr + R (n = 88; 95% CI, 13.9-23.0; P < .0001). Similarly, after 24 months of therapy, bone marrow MRD levels were also significantly lower in Ibr + R–treated patients. At this time point, the mean MRD level with Ibr alone was 19.8% (n = 75; 95% CI, 15.5-24.0) and 12.2% for patients receiving Ibr + R (n = 64; 95% CI, 7.5-16.8; P = .0180). Residual disease levels in various CLL subgroups after 12 and 24 months of therapy are depicted in supplemental Table 10. A total of 6 patients became bone marrow MRD-negative: 5 patients who had received Ibr + R and 1 who received Ibr alone. Three of the 6 patients who became MRD-negative in the bone marrow were treatment-naive, and all of these patients had received Ibr + R.
Time to remission and kinetics of CLL blood indices during treatment with Ibr or Ibr + R. (A) Number of patients in CR at 12 and 24 months. (B) Bone marrow CLL disease levels, quantified by using flow cytometry, at baseline and after 12 and 24 months of treatment. (C) Trended mean ± SD ALC in patients treated with Ibr (blue line) or with Ibr + R (green line). (D) Trended mean ± SD β2-microglobulin levels in patients treated with Ibr or with Ibr + R. (E) Trended mean ± SD hemoglobin levels in patients treated with Ibr or with Ibr + R. (F) Trended mean ± SD platelet counts in patients treated with Ibr or with Ibr + R. CR/CRI, CRs with incomplete count recovery.
Time to remission and kinetics of CLL blood indices during treatment with Ibr or Ibr + R. (A) Number of patients in CR at 12 and 24 months. (B) Bone marrow CLL disease levels, quantified by using flow cytometry, at baseline and after 12 and 24 months of treatment. (C) Trended mean ± SD ALC in patients treated with Ibr (blue line) or with Ibr + R (green line). (D) Trended mean ± SD β2-microglobulin levels in patients treated with Ibr or with Ibr + R. (E) Trended mean ± SD hemoglobin levels in patients treated with Ibr or with Ibr + R. (F) Trended mean ± SD platelet counts in patients treated with Ibr or with Ibr + R. CR/CRI, CRs with incomplete count recovery.
Bone marrow MRD level (%)
| Treatment . | Ibr . | Ibr + R . | Ibr vs Ibr + R . | |||
|---|---|---|---|---|---|---|
| n . | LSMEAN (95% CI) . | n . | LSMEAN (95% CI) . | LSMEAN Difference . | P . | |
| 12 mo | 89 | 34.4 (29.9-39.0) | 88 | 18.5 (13.9-23.0) | 16.0 (9.6-22.4) | <.0001 |
| 24 mo | 75 | 19.8 (15.5-24.0) | 64 | 12.2 (7.5-16.8) | 7.6 (1.3-13.9) | .0180 |
| Treatment . | Ibr . | Ibr + R . | Ibr vs Ibr + R . | |||
|---|---|---|---|---|---|---|
| n . | LSMEAN (95% CI) . | n . | LSMEAN (95% CI) . | LSMEAN Difference . | P . | |
| 12 mo | 89 | 34.4 (29.9-39.0) | 88 | 18.5 (13.9-23.0) | 16.0 (9.6-22.4) | <.0001 |
| 24 mo | 75 | 19.8 (15.5-24.0) | 64 | 12.2 (7.5-16.8) | 7.6 (1.3-13.9) | .0180 |
LSMEAN is derived based on the analysis of covariance model with effect for treatment and baseline MRD level as a covariate. LSMEAN, least squares mean.
Laboratory markers
Trended ALC for patients receiving Ibr or Ibr + R are displayed in Figure 3C. Patients receiving combination therapy had lower ALC during the first year of treatment than patients receiving Ibr alone; consequently, the median time to normalization of the ALC to <4000/μL was 24 weeks in patients taking Ibr + R (range, 1-144 weeks; n = 66) and 48 weeks in patients receiving Ibr alone (range, 2-192 weeks; n = 64) (supplemental Table 11). Descriptive statistics of ALC in both treatment groups are summarized in supplemental Table 12. The mean ± SD β2-microglobulin level before therapy was 4.1 ± 2.1 mg/L in patients taking Ibr alone (n = 100) and 4.1 ± 1.9 mg/L in patients taking Ibr + R (n = 102), which decreased and eventually normalized during therapy; there were no discernible differences between groups (Figure 3D; supplemental Table 13). Sustained improvement in hemoglobin levels and platelet counts were observed after 4 weeks of therapy, also without any discernible differences between groups (Figure 3E-F).
Immunoglobulin and T-cell changes
We noted a decline in immunoglobulin G (IgG) levels in patients treated with Ibr and Ibr + R. For example, mean IgG levels in Ibr-treated patients declined from 754.4 ± 56.4 mg/dL (n = 101) before therapy to 656.4 ± 38.3 mg/dL after 24 months of treatment (n = 75) and to 600 ± 72.1 mg/dL after 36 months of treatment (n = 21). In Ibr + R–treated patients, mean IgG levels declined from 652.3 ± 33.3 mg/dL at baseline (n = 104) to 563.1 ± 30.4 mg/dL after 24 months (n = 62) and to 431.5 ± 35.4 mg/dL after 36 months (n = 19). We also noted a decrease in immunoglobulin M levels in Ibr + R–treated patients. In contrast, immunoglobulin A levels increased over time (supplemental Figure 2; supplemental Table 14). These changes in immunoglobulin levels are in accordance with previous reports by us14 as well as others.22
T cells and T-cell subset numbers also changed during treatment with Ibr and Ibr + R. In Ibr-treated patients, elevated numbers of CD3+ T cells before treatment dropped from a mean of 3469 ± 322 cells/μL (n = 74) to 2945 ± 364 cells/μL after 6 months (n = 63), and further declined to 1549 ± 155 cells/μL after 12 months (n = 48) and to 941 ± 136 cells/μL after 24 months (n = 12) (supplemental Table 15). In patients receiving Ibr + R, there was a significantly more rapid decline in CD3+ T-cell numbers from 3496 ± 473 cells/μL before therapy (n = 76) to 1910 ± 200 cells/μL after 6 months (n = 57; P = .017), which then plateaued at 1629 ± 185 cells/μL after 12 months (n = 44) and at 1793 ± 451 cells/μL after 24 months (n = 8). Similarly, CD4+ and CD8+ T-cell numbers declined more rapidly in patients taking Ibr + R than in patients taking Ibr alone (supplemental Figure 3B-C).
Safety
Treatment was generally well tolerated, and toxicities were consistent with those previously described in patients receiving long-term therapy with Ibr.23 Grade 3/4 treatment-emergent adverse events (TEAEs) were reported in 64% of patients receiving Ibr and in 65% of patients receiving Ibr + R. The most frequent grade 3/4 TEAEs were arterial hypertension in 30.8% of Ibr-treated patients and in 31.7% of Ibr + R–treated patients, and grade 3/4 infections in 22.2% of patients receiving Ibr and in 15.6% of patients receiving Ibr + R. Grade 3/4 neutropenia occurred in 9.6% of patients receiving Ibr and in 13.5% of patients receiving Ibr + R, and grade 3/4 thrombocytopenia occurred in 4.8% of patients receiving Ibr and in 6.7% of patients receiving Ibr + R. Atrial fibrillation (any grade) occurred in 12.5% of Ibr-treated patients and in 9.6% of Ibr + R–treated patients; grade 3/4 atrial fibrillation was reported in 7.7% of patients receiving Ibr and in 5.8% of patients receiving Ibr + R. Bruising (any grade) was noted in 25% of Ibr-treated patients and in 24% of Ibr + R–treated patients; none of those events were grade 3/4. Details regarding TEAEs in all patients are summarized in supplemental Tables 16 and 17.
Discussion
Treatment of patients with CLL has undergone fundamental changes during the past few years due to the availability of new targeted agents, including Ibr. Because of the high response rates, durability of responses, and favorable tolerability, Ibr has become a frequently used treatment alternative to chemotherapy-based regimens for patients with CLL and is considered first-choice therapy for CLL patients with higher risk disease.1 However, when used as a single agent, Ibr induces CRs only in a minority of patients.23 The addition of anti-CD20 antibodies to the chemotherapy regimen as chemoimmunotherapy5,17,18,24 resulted in improved ORR, prolonged PFS, and OS.5,17 When combined with novel agents such as idelalisib3 or venetoclax,7 rituximab improves response rates. However, data on survival benefit (PFS or OS) from anti-CD20 antibody combinations with novel agents are lacking. With a median follow-up of 36 months, we observed no differences in PFS, OS, or ORR between the study arms (Figure 2). A slightly higher CR rate was noted in Ibr + R–treated patients, which was more noticeable in high-risk patients with del17p, and especially in previously untreated patients (Figure 1), in whom the addition of rituximab increased the CR rate from 20% to 50%; interestingly, one-half of these patients had undetectable MRD. However, due to the relatively small number of patients in these subset analyses, none of these differences reached statistical significance.
Regarding the lack of PFS and OS benefit from the addition of rituximab to Ibr, we must consider the fact that patients in both treatment arms overall did very well, with only a small number of patients developing resistance and disease progression during the 3 years of follow-up, which is reassuring, given the higher risk population enrolled. A recent cross-trial comparison of several pivotal phase 3 trials in treatment-naive patients with CLL concluded that patients receiving Ibr had the longest PFS compared with patients receiving any other type of therapy (ie, fludarabine, cyclophosphamide, and rituximab; BR; chlorambucil + anti-CD20 monoclonal antibodies).25 Accordingly, the HELIOS (Ibrutinib Combined with Bendamustine and Rituximab Compared with Placebo, Bendamustine, and Rituximab for Previously Treated Chronic Lymphocytic Leukaemia or Small Lymphocytic Lymphoma) trial,15 which reported significantly improved PFS from the addition of Ibr to BR in relapsed/refractory CLL, did not show any added survival benefit from BR in the BR + Ibr combination, compared with single-agent Ibr in another cross-trial comparison, with its inherent limitations.26 At this time, however, we cannot exclude the possibility that the addition of CD20 antibodies in the CLL frontline therapy setting may affect PFS, as could be inferred from the high CR rate seen in this population (Figure 1). An ongoing clinical trial that compares Ibr vs Ibr + R or fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in previously untreated CLL27 will likely answer this question in the near future.
Regarding depth of remission, serial bone marrow MRD testing found that patients who received Ibr + R had lower residual disease levels than those treated with Ibr alone (Table 2). Accordingly, patients treated with combination therapy had faster resolution of their peripheral blood lymphocytosis (Figure 3C). Interestingly, elevated CD4 and CD8 T-cell counts decreased and then normalized significantly faster in patients receiving Ibr + R, mirroring the faster achievement of remission in this group of patients; this outcome also supports the concept of interdependence between the CLL clone and supportive T-cell clones28,29 that vanish during Ibr treatment, as shown by T-cell receptor deep sequencing.28
A potential concern regarding Ibr + R therapy, based on preclinical studies, has been related to a potential antagonism between Ibr and rituximab, based on the off-target activity of Ibr toward interleukin-2 inducible tyrosine kinase,24 which is expressed in natural killer cells and T cells. In preclinical models, it was noted that Ibr can antagonize rituximab’s antilymphoma activity, based on Ibr’s interference with Fc receptor-stimulated antibody-dependent cell-mediated cytotoxicity (ADCC).25 However, the relative contribution and importance of natural killer cells, monocytes, or other effector cells for CD20 antibody–mediated killing of B cells remains controversial. The more rapid clearance of CLL cells from the peripheral blood and the bone marrow in patients receiving combination therapy in this trial indicate that Ibr does not play an antagonistic role when combined with rituximab in the clinical setting, although ADCC was not formally analyzed, and it may be that ADCC-independent agents have higher efficacy when combined with Ib. The glycoengineered type II anti-CD20 antibody obinutuzumab (GA101) has higher direct cytotoxicity and ADCC in preclinical assays, compared with rituximab,30 and seems to be superior to rituximab when combined with chlorambucil in untreated elderly patients with CLL.5 Ongoing clinical trials will determine whether these benefits also apply to obinutuzumab when combined with Ibr and other targeted agents.
Regarding the broader CLL treatment landscape, the role of anti-CD20 antibodies as combination partner for the novel agents remains controversial. Rituximab is routinely combined with the phosphatidylinositol 3-kinase inhibitor idelalisib, based on higher response rates3 rather than improvement in PFS or OS. Similarly, venetoclax in combination with rituximab is now increasingly used,7 also because of higher response rates, without proven impact on survival. Based on the data in the present study, single-agent Ibr remains the standard of care for patients with CLL in the relapsed/refractory disease setting. The noted faster clearance of the disease from blood and marrow by the addition of rituximab may, however, be beneficial in certain patients with high white blood cell counts and/or disease-related symptoms, in whom the achievement of an earlier remission could be desirable. It could also become a useful strategy to achieve the best response at an early time point, in the context of new clinical trial concepts, in which patients receive limited-time treatment to best response, with the aim of avoiding long-term toxicity and/or development of resistance.
In conclusion, this trial shows that the addition of rituximab to Ibr therapy for patients with relapsed/refractory CLL with inclusion of previously untreated del17p patients did not improve PFS or OS and did not significantly increase overall response or CR rates. Although patients treated with the combination therapy achieved remissions earlier and had lower levels of residual disease, these benefits do not at this time justify the use of combination therapy in this group of patients and, consequently, Ibr monotherapy should remain standard of care. Ongoing randomized studies comparing Ibr monotherapy with Ibr + R, or chemoimmunotherapy in patients with untreated CLL, will determine whether CD20 antibodies may have greater impact in the frontline disease setting.
Primary results were presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families, as well as the study investigators and coordinators at MD Anderson Cancer Center. The study was supported by Pharmacyclics LLC, an AbbVie Company; Leukemia & Lymphoma Society grant ID 2166-14; and MD Anderson Cancer Center Support grant CA016672.
Authorship
Contribution: J.A.B. designed and supervised the trial, analyzed the data, and wrote the paper; A.F., N.J., T.K., Z.E., M.O., M.A., P.T., H.K., S.O., W.G.W., and M.J.K. contributed to the trial design, clinical patient management, sample collection, and clinical data analysis, and reviewed and approved the paper; J.J. analyzed and supervised flow cytometry analyses; and M.S., E.K., G.M.N.-G., X.H., J.L., M.C., F.C., and T.M. performed data collection and statistical analyses of the data.
Conflict-of-interest disclosure: J.A.B., N.J., and S.O. received research funding from Pharmacyclics LLC. J.A.B. and N.J. are consultants for Janssen Pharmaceuticals, Inc. N.J. and P.T. have served as consultants for AbbVie and Pharmacyclics. J.L., M.C., and F.C. are employees of Pharmacyclics LLC. The remaining authors declare no competing interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org
REFERENCES
Author notes
W.G.W., A.F., and M.J.K. contributed equally as senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal