Abstract
The liver orchestrates systemic iron balance by producing and secreting hepcidin. Known as the iron hormone, hepcidin induces degradation of the iron exporter ferroportin to control iron entry into the bloodstream from dietary sources, iron recycling macrophages, and body stores. Under physiologic conditions, hepcidin production is reduced by iron deficiency and erythropoietic drive to increase the iron supply when needed to support red blood cell production and other essential functions. Conversely, hepcidin production is induced by iron loading and inflammation to prevent the toxicity of iron excess and limit its availability to pathogens. The inability to appropriately regulate hepcidin production in response to these physiologic cues underlies genetic disorders of iron overload and deficiency, including hereditary hemochromatosis and iron-refractory iron deficiency anemia. Moreover, excess hepcidin suppression in the setting of ineffective erythropoiesis contributes to iron-loading anemias such as β-thalassemia, whereas excess hepcidin induction contributes to iron-restricted erythropoiesis and anemia in chronic inflammatory diseases. These diseases have provided key insights into understanding the mechanisms by which the liver senses plasma and tissue iron levels, the iron demand of erythrocyte precursors, and the presence of potential pathogens and, importantly, how these various signals are integrated to appropriately regulate hepcidin production. This review will focus on recent insights into how the liver senses body iron levels and coordinates this with other signals to regulate hepcidin production and systemic iron homeostasis.
Roles of iron in biology
Iron is an essential element required for numerous fundamental cellular and organismal processes, including oxygen transport, mitochondrial respiration, nucleic acid replication, intermediary and xenobiotic metabolism, host defense, and cell signaling. These biological functions depend on iron’s property as a transition metal to readily donate or accept electrons to participate in oxidation-reduction reactions. Iron performs these essential functions as a constituent of proteins in the form of heme, iron sulfur clusters, or other functional groups, thereby targeting and limiting its reactivity. However, when excess “free” iron is present, this redox cycling can also catalyze the production of free radicals, which can damage DNA, proteins, and lipids. To meet the dual challenge of providing adequate iron to perform essential functions while limiting the toxicity of excess iron, organisms have evolved specialized proteins and homeostatic mechanisms to tightly control iron availability.
Systemic iron homeostasis
At a systemic level, iron homeostasis is maintained by balancing iron supply with iron utilization and losses (reviewed in Dev and Babitt1 and Muckenthaler et al2 ). In humans, ∼20 mg iron is used per day to generate 200 billion erythrocytes, accounting for 80% of daily iron needs. Erythrocytes and other cells obtain iron from the plasma, which contains only 2 to 4 mg iron and therefore must be turned over every few hours to meet the daily requirement. Iron is supplied to the plasma by reticuloendothelial macrophages that salvage iron from aged erythrocytes (20-25 mg daily) and duodenal enterocytes that absorb dietary iron (1-2 mg daily), with the remainder provided by body stores. Iron losses (∼1-2 mg/day) occur mainly through desquamation and blood loss in an unregulated process. Since iron losses are unregulated, iron homeostasis is mainly controlled by regulating iron supply.
The major suppliers of iron to the plasma are duodenal enterocytes and iron-recycling macrophages (reviewed in Muckenthaler et al2 ). Dietary elemental iron is transported across the enterocyte apical membrane by divalent metal transporter 1 (DMT1) after reduction by ferriductases such as duodenal cytochrome B.3-6 Alternative mechanisms exist for heme iron uptake, but the specific transport mechanisms are unknown. Reticuloendothelial macrophages phagocytose aged erythrocytes for degradation in phagolysosomes, followed by iron release by heme oxygenase 1. In both enterocytes and macrophages, imported iron can be either used for the cell’s own metabolic processes, stored in an inert form in ferritin, or exported into the plasma for use by other tissues. Elemental iron export is coupled to oxidation by the ferroxidases hephaestin and ceruloplasmin for loading onto the plasma iron carrier transferrin for delivery to erythrocyte precursors and other tissues.7-9
Regulation of systemic iron homeostasis by the hepcidin-ferroportin axis
Physiologic studies dating back to the mid-1900s supported the existence of several regulators that modulate the iron supply to maintain stable iron stores (stores regulator), provide adequate iron for erythropoiesis (erythroid regulator), and limit iron availability to pathogenic microorganisms (inflammatory regulator) (reviewed in Finch10 ). In 2000-2001, 2 key proteins were identified, hepcidin11-13 and ferroportin,14-16 which began to unravel the puzzle of how communication was coordinated among organs involved in iron utilization, recycling, storage, and acquisition to maintain systemic iron homeostasis. Hepcidin is a 25-amino-acid hormone that is synthesized and secreted predominantly by the liver, circulates in the bloodstream, and is excreted by the kidneys.11-13 A key role for hepcidin in iron homeostasis regulation was recognized when mutations or knockout of this gene were associated with the iron overload disorder hereditary hemochromatosis (HH), characterized by excessive dietary iron absorption and iron release from macrophage stores leading to serum and tissue iron overload with consequent organ dysfunction.17,18 Conversely, hepcidin excess in transgenic mice or humans with hepcidin-expressing adenomas resulted in iron deficiency anemia.19,20 The role of hepcidin as an effector for the stores, erythroid, and inflammatory regulators was suggested by the upregulation of hepcidin expression by iron loading and inflammation12 and its suppression by erythropoietic drive.21
Coincident with the discovery of hepcidin, ferroportin was identified as an iron transport protein expressed on the basolateral surface of duodenal enterocytes, reticuloendothelial macrophages, hepatocytes, and placental syncitiotrophoblasts, where it plays a key role in iron export into the plasma.14-16 Inactivating ferroportin mutations in zebrafish resulted in iron deficiency anemia due to inadequate maternal iron transport to the fetus.15 Conditional ferroportin deletion from intestinal enterocytes, macrophages, and hepatocytes in mice cemented the important functional role of ferroportin in dietary iron absorption and iron mobilization to maintain systemic iron homeostasis.22-24 Recent work has also discovered a local role for ferroportin in other cell types, including cardiac myocytes25 and erythroid cells,26 to prevent toxicity from iron excess. Ferroportin mutations in humans lead to 2 distinct phenotypes, one resembling classical HH and the other (so-called ferroportin disease) manifesting in an autosomal-dominant fashion by iron loading predominantly in reticuloendothelial macrophages with a tendency toward reduced plasma iron and anemia.27-29
Shortly after their discovery, hepcidin and ferroportin were functionally linked when it was recognized that hepcidin binds ferroportin and induces its internalization and degradation.30 Data from crystal structure of a putative bacterial ferroportin homolog, computational modeling, patient mutations, and in vitro studies have refined our understanding of how ferroportin transports iron and how its activity is regulated by hepcidin.31-37 A member of the major facilitator superfamily of transporters, ferroportin is a 12-transmembrane domain protein organized into 2 lobes connected by a large cytoplasmic loop.31,32 In the current model, the 2 lobes form a central cavity containing a metal-binding site, and transport activity occurs by a conformational change from an inward-facing state open intracellularly to an outward-facing state open extracellularly.33 The N terminus of hepcidin34,35 binds to the central cavity of ferroportin, where it interacts with up to 4 helices.36 Hepcidin binding triggers ferroportin to undergo a conformation change, ubiquitination of multiple lysine residues on the cytoplasmic loop, endocytosis, and degradation.37,38 Hepcidin binding also occludes ferroportin and interferes with iron export independent of endocytosis activity.36 These studies provide a molecular explanation for the pathophysiology of HH due to hepcidin mutations and the differing patient phenotypes resulting from ferroportin mutations. Loss of hepcidin function leads to iron overload as a consequence of unregulated ferroportin activity, hyperabsorption of dietary iron, and excess iron release from body stores. Ferroportin mutations that interfere with hepcidin binding or the conformational change required for endocytosis and degradation result in a gain-of-function phenotype resembling classical HH. Ferroportin mutations that interfere with its localization or iron export function result in a loss-of-function phenotype and ferroportin disease. An unresolved question is why loss-of-function mutations act in a dominant-negative fashion to cause autosomal-dominant disease.
The ability of hepcidin to control ferroportin expression and activity also provides a molecular explanation for how hepcidin functions as the key hormone to coordinate signals from iron, erythropoietic drive, and inflammation to control systemic iron homeostasis (Figure 1). Iron loading increases hepcidin expression, thereby inhibiting ferroportin and limiting iron entry into the bloodstream to maintain steady-state iron levels. Hepcidin is regulated not only by the classically described iron “stores regulator” but also by acute increases in plasma iron, even when stores are unchanged.39,40 This explains why the plasma iron concentration remains relatively stable despite its rapid turnover. Inflammation also induces hepcidin, thereby inhibiting ferroportin to limit iron availability to pathogens. Conversely, iron deficiency and erythropoietic drive suppress hepcidin, thereby increasing ferroportin to increase iron availability for erythropoiesis and other essential functions.
Systemic iron homeostasis regulation by hepcidin and ferroportin. Hepcidin and ferroportin control iron entry into the circulation from dietary sources and body stores to maintain systemic iron homeostasis. Iron deficiency and erythropoietic demand suppress hepcidin production by hepatocytes (left). In the absence of hepcidin, ferroportin is stabilized on the basolateral surface of duodenal enterocytes, iron-recycling macrophages, and hepatocytes, where it transports iron (Fe) from the intracellular space to the plasma for loading onto transferrin (TF) and delivery to red blood cells and other tissues. Iron loading and inflammation stimulate hepatocyte hepcidin production and secretion into the circulation, where it binds to ferroportin to cause its ubiquitination (U), endocytosis, and degradation (right). Hepcidin binding also interferes with ferroportin export activity independent of endocytosis. Hepcidin thereby limits iron overload and sequesters iron from invading pathogens.
Systemic iron homeostasis regulation by hepcidin and ferroportin. Hepcidin and ferroportin control iron entry into the circulation from dietary sources and body stores to maintain systemic iron homeostasis. Iron deficiency and erythropoietic demand suppress hepcidin production by hepatocytes (left). In the absence of hepcidin, ferroportin is stabilized on the basolateral surface of duodenal enterocytes, iron-recycling macrophages, and hepatocytes, where it transports iron (Fe) from the intracellular space to the plasma for loading onto transferrin (TF) and delivery to red blood cells and other tissues. Iron loading and inflammation stimulate hepatocyte hepcidin production and secretion into the circulation, where it binds to ferroportin to cause its ubiquitination (U), endocytosis, and degradation (right). Hepcidin binding also interferes with ferroportin export activity independent of endocytosis. Hepcidin thereby limits iron overload and sequesters iron from invading pathogens.
After discovery of the hepcidin-ferroportin axis, it quickly became clear that excessive or insufficient hepcidin production plays a central role in most major disorders of systemic iron homeostasis. Whereas hepcidin suppression by anemia or hypoxia has a physiologic role to increase iron availability for erythropoiesis, excessive hepcidin suppression by ineffective erythropoiesis contributes to pathologic iron overload in thalassemias and other iron-loading anemias. Whereas hepcidin induction may be protective to sequester iron during infection, excessive hepcidin induction contributes to iron-restricted erythropoiesis and anemia in chronic inflammatory diseases. The inability of iron loading to appropriately upregulate hepcidin leads to HH due not only to mutations in hepcidin or ferroportin themselves but also to other genetic causes. The inability of iron deficiency to appropriately suppress hepcidin leads to iron-refractory iron deficiency anemia (IRIDA). Targeting hepcidin and/or ferroportin function has therefore become an attractive new therapeutic strategy for these iron disorders, with efficacy demonstrated in many animal models and numerous candidates now being translated to clinical studies.41,42 Understanding the genetic and molecular basis of these iron disorders has also yielded important insights into how hepcidin is regulated by its various stimuli and has opened up additional therapeutic opportunities that target hepcidin production.
Hepcidin regulation by iron: lessons from HH and IRIDA
In addition to hepcidin and gain-of-function ferroportin mutations, mutations in several other genes, including HFE, transferrin receptor 2 (TFR2), and hemojuvelin (HJV), lead to HH.43 All patients and animal models with a global or hepatocyte-specific knockout or mutation in HFE, TFR2, or HJV exhibit hepcidin deficiency and iron overload, suggesting that the key function of all of these proteins is in regulating hepatic hepcidin production in response to iron.44-52 Mutations in transmembrane serine protease 6 (TMPRSS6) lead to an opposite phenotype of IRIDA as a consequence of hepcidin excess, suggesting that TMPRSS6 plays in an important functional role to suppress hepcidin production in response to iron deficiency.53-55
Hepcidin regulation by HJV and the bone morphogenetic protein (BMP)-SMAD pathway
In 2006, HJV, like its repulsive guidance molecule family homologs RGMA and RGMB,56,57 was recognized to function as a coreceptor for the BMP-SMAD pathway.58 This led to the discovery of the BMP-SMAD signaling pathway as a major transcriptional regulator of hepcidin expression in hepatocytes58 (Figure 2). HJV binds directly to BMP ligands and facilitates the formation of an active signaling complex containing BMP type I and type II receptors.58-60 This signaling complex phosphorylates receptor-activated SMADs (R-SMADs), which bind to the common mediator SMAD4 and translocate to the nucleus, where they bind 2 specific elements on the hepcidin promoter61,62 to induce transcription. BMP signaling may also impact hepcidin expression by epigenetic mechanisms.63
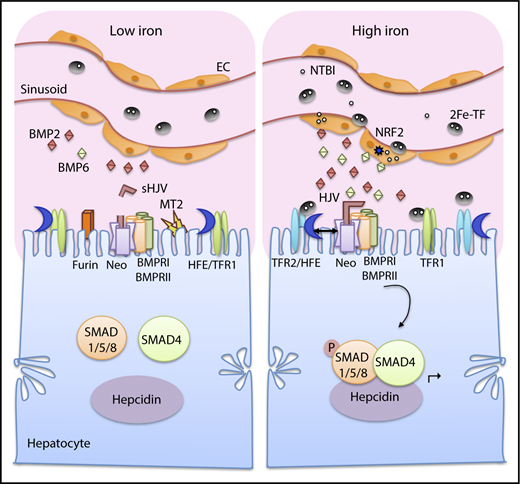
Current model of iron sensing in the liver to regulate hepcidin production. Both tissue iron and plasma iron levels are sensed by the liver to regulate hepcidin production. Iron loading increases transferrin-bound iron (2Fe-TF) and non–transferrin-bound iron (NTBI) in the circulation (right). Iron is taken up by liver endothelial cells (ECs), which play an important role in sensing tissue iron levels. Iron loading in ECs increase BMP6 and, to a lesser extent, BMP2 expression. BMP6 is partially regulated by nuclear factor (erythroid-derived 2)-like 2 (NRF2), which is activated by iron-induced reactive oxygen species. The increased BMP2 and BMP6 bind to HJV on the hepatocyte membrane and facilitate the formation of a signaling complex consisting of BMP type I and type II receptors (BMPR1 and BMPRII). Neogenin (Neo), a scaffold protein binding to HJV, assists with the signaling complex formation and localization. The signaling complex phosphorylates SMAD1/5/8, which binds to SMAD4 and translocates to the nucleus to induce hepcidin (Hamp) transcription. Plasma iron loading is also sensed directly by hepatocytes, where 2Fe-TF binds to TFR1 and TFR2 on the hepatocyte membrane, favoring the displacement of HFE from TFR1 and the interaction between HFE and TFR2. Both HFE and TFR2 stimulate hepcidin expression via a functional interaction with the SMAD pathway, possibly by forming a complex with HJV and stabilizing BMP type I receptor ALK3. Under low-iron conditions, BMP2 and BMP6 ligand production is reduced in ECs (left). HFE binds to TFR1, whose expression levels increase, whereas TFR2 levels decrease. Iron deficiency also increases TMPRSS6/matriptase-2 (MT2) and furin, which cleave membrane HJV to generate soluble HJV (sHJV). Suppression of BMP expression, HJV cleavage from the membrane, sequestration of HFE with TFR1, and reduced TFR2 expression all diminish BMP-SMAD signaling, thus suppressing hepcidin expression.
Current model of iron sensing in the liver to regulate hepcidin production. Both tissue iron and plasma iron levels are sensed by the liver to regulate hepcidin production. Iron loading increases transferrin-bound iron (2Fe-TF) and non–transferrin-bound iron (NTBI) in the circulation (right). Iron is taken up by liver endothelial cells (ECs), which play an important role in sensing tissue iron levels. Iron loading in ECs increase BMP6 and, to a lesser extent, BMP2 expression. BMP6 is partially regulated by nuclear factor (erythroid-derived 2)-like 2 (NRF2), which is activated by iron-induced reactive oxygen species. The increased BMP2 and BMP6 bind to HJV on the hepatocyte membrane and facilitate the formation of a signaling complex consisting of BMP type I and type II receptors (BMPR1 and BMPRII). Neogenin (Neo), a scaffold protein binding to HJV, assists with the signaling complex formation and localization. The signaling complex phosphorylates SMAD1/5/8, which binds to SMAD4 and translocates to the nucleus to induce hepcidin (Hamp) transcription. Plasma iron loading is also sensed directly by hepatocytes, where 2Fe-TF binds to TFR1 and TFR2 on the hepatocyte membrane, favoring the displacement of HFE from TFR1 and the interaction between HFE and TFR2. Both HFE and TFR2 stimulate hepcidin expression via a functional interaction with the SMAD pathway, possibly by forming a complex with HJV and stabilizing BMP type I receptor ALK3. Under low-iron conditions, BMP2 and BMP6 ligand production is reduced in ECs (left). HFE binds to TFR1, whose expression levels increase, whereas TFR2 levels decrease. Iron deficiency also increases TMPRSS6/matriptase-2 (MT2) and furin, which cleave membrane HJV to generate soluble HJV (sHJV). Suppression of BMP expression, HJV cleavage from the membrane, sequestration of HFE with TFR1, and reduced TFR2 expression all diminish BMP-SMAD signaling, thus suppressing hepcidin expression.
Mouse genetic studies have identified the key molecular players in the BMP-SMAD pathway that work in concert with HJV to regulate hepcidin and systemic iron homeostasis: BMP664,65 and BMP2 ligands,66,67 ALK2 and ALK3 type I receptors,68 ACVR2A and BMPR2 type II receptors,69 R-SMADs 1 and 5,70 and SMAD4,63 since global or tissue-specific knockout of these molecules resulted in hepcidin deficiency and HH in mice (Table 1). SMAD7 and SMAD6 are inhibitory SMADs that are induced by BMP-SMAD signaling and feedback to inhibit the pathway by interfering with type I receptor function or SMAD complex formation. Hepatocyte Smad7 knockout mice exhibited hepcidin excess and mild iron deficiency anemia consistent with a physiologic role in inhibiting hepcidin expression in vivo71 (Table 1). A similar inhibitory effect of Smad6 on hepcidin expression was also demonstrated in vitro.72 Neogenin is another HJV-interacting protein that binds a distinct domain from BMP ligands60 and may function as a scaffold protein to assist complex formation and localization to modulate signaling.73-75 Neogenin mutant mice exhibited impaired BMP signaling, hepcidin deficiency, and iron overload consistent with an in vivo role76 (Table 1). Whereas most of these proteins function to regulate hepcidin directly in hepatocytes, BMP2 and BMP6 ligands originate from liver endothelial cells, where they have paracrine effects on HJV and the BMP receptor complex.67,77 The relevance of these molecular players in humans is corroborated by reports of higher ferritin in HFE hemochromatosis patients with a common BMP2 polymorphism78 and iron overload in patients with heterozygous mutations in the BMP6 prodomain,79,80 although the causal role of the human BMP6 mutations has been questioned.81 Building on these molecular discoveries, pharmacologic BMP-SMAD pathway inhibitors lowered hepcidin and increased serum and tissue iron in normal mice and reversed hepcidin excess, iron-restricted erythropoiesis, and anemia in animal models of anemia of inflammation.64,66,82-85 Conversely, exogenous BMP2 or BMP6 induced hepcidin and reduced serum iron in normal mice and ameliorated hemochromatosis in Hfe−/− mice.58,64,86 These data support the therapeutic possibility of targeting BMP-SMAD signaling in diseases of hepcidin excess or deficiency.
Phenotype of mice with a single knockout of key players in liver iron sensing
| Single mutant . | Hepcidin . | Liver iron . | Extrahepatic iron loading . | Anemia . | References . |
|---|---|---|---|---|---|
| Ligand | |||||
| Bmp6 | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 64,65,77,90 |
| Bmp2* | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 66,67 |
| Cell membrane–sensing molecules and receptors | |||||
| Hjv | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 47,90 |
| Hfe or β2m | ↓ | ↑↑ | No | No | 107 |
| Tfr2 | ↓↓ | ↑↑↑ | No | No | 107 |
| Alk2† | ↓ | ↑ | n/a | No | 68 |
| Alk3† | ↓↓↓ | ↑↑↑↑ | n/a | No | 68 |
| Acvr2a or Bmpr2† | → | → | n/a | No | 69 |
| Tmprss6 | ↑ | ↓ | No | Yes | 109,112 |
| Neogenin | ↓ | ↑↑ | n/a | No | 76 |
| Intracellular molecules | |||||
| Smad1 or Smad5† | 12 d ↓ | → | n/a | No | 70 |
| Adult→ | |||||
| Smad4† | ↓↓↓ | ↑↑↑↑ | Yes‡ | No | 63 |
| Smad7† | ↑ | ↓ | n/a | Yes | 71 |
| Single mutant . | Hepcidin . | Liver iron . | Extrahepatic iron loading . | Anemia . | References . |
|---|---|---|---|---|---|
| Ligand | |||||
| Bmp6 | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 64,65,77,90 |
| Bmp2* | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 66,67 |
| Cell membrane–sensing molecules and receptors | |||||
| Hjv | ↓↓↓ | ↑↑↑↑ | Yes (M>F) | No | 47,90 |
| Hfe or β2m | ↓ | ↑↑ | No | No | 107 |
| Tfr2 | ↓↓ | ↑↑↑ | No | No | 107 |
| Alk2† | ↓ | ↑ | n/a | No | 68 |
| Alk3† | ↓↓↓ | ↑↑↑↑ | n/a | No | 68 |
| Acvr2a or Bmpr2† | → | → | n/a | No | 69 |
| Tmprss6 | ↑ | ↓ | No | Yes | 109,112 |
| Neogenin | ↓ | ↑↑ | n/a | No | 76 |
| Intracellular molecules | |||||
| Smad1 or Smad5† | 12 d ↓ | → | n/a | No | 70 |
| Adult→ | |||||
| Smad4† | ↓↓↓ | ↑↑↑↑ | Yes‡ | No | 63 |
| Smad7† | ↑ | ↓ | n/a | Yes | 71 |
Levels of hepcidin expression and tissue iron loading are subject to variation based on background strains, ages, and analytical variations in different studies.
F, female; M, male; n/a, data not available.
Endothelial conditional knockout.
Hepatocyte conditional knockout.
Study did not indicate sex of mice used.
BMP ligands and receptors function in a multimeric complex containing a dimeric ligand, 2 type I receptors, and 2 type II receptors. In some contexts, heterodimeric ligands or receptor complexes (containing 2 different ligands or type I receptors) are critical for biological function.87,88 Although individual components have been identified, the full nature of the BMP-HJV-receptor signaling complex(es) for hepcidin regulation remains unknown. Whereas HJV enhances BMP induction of hepcidin in cell culture systems and animals, HJV is not required for BMP2 or BMP6 signaling, since these ligands can still induce hepcidin, albeit less potently, in Hjv−/− primary hepatocytes.58,89 Moreover, mice with a knockout of both Bmp6 and Hjv have a stronger iron overload phenotype than single Hjv knockout mice90 (Table 2). Interestingly, the crystal structure of BMP2 in complex with HJV reveals that HJV binds to the same interface as the type I receptor and even competes for type I receptor binding in cell-free binding assays.60 Since type I receptors are required for R-SMAD phosphorylation, how HJV facilitates rather than inhibits signaling in cell culture systems and in vivo is unclear. One hypothesis suggests that HJV enhances BMP binding on the cell surface, and after internalization, type I receptors replace HJV in the acidic endosomal compartment that favors BMP type I receptor interactions over BMP-HJV interactions.60 This hypothesis awaits validation by functional studies. HJV may also interact with other proteins that modulate optimal BMP-SMAD signal transduction in response to iron, such as neogenin60 (as discussed above) or HFE, TFR2, and TMPRSS6 (as discussed below).91,92 Another open question involves the relative roles of individual BMP ligands and receptors, whether they are activated under different conditions, have different signaling efficiency, or involve heteromeric ligand or receptor complexes. Type II receptors have completely redundant functions, since single hepatocyte knockout of either Acvr2a or Bmpr2 did not impact iron homeostasis, whereas double knockout caused HH.69 In contrast, hepatocyte knockout of either type I receptor caused HH, but Alk3 knockout had a stronger phenotype than Alk2 knockout, suggesting at least some nonoverlapping functions68 Endothelial knockout of either Bmp2 or Bmp6 also caused HH, consistent with nonredundant roles66,67,77 ; however, severity was not directly comparable due to different genetic backgrounds (Tables 1 and 2).
Phenotype of mice with double vs single knockout of key players in liver iron sensing
| Double mutant . | Compared with . | Hamp levels . | Liver iron . | Extrahepatic iron loading . | Anemia . | References . |
|---|---|---|---|---|---|---|
| Bmp6 × Hjv | Bmp6 or Hjv | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑↑ (M) → | No | 90,107 |
| Bmp6 × β2m | β2m | ↓↓↓ | ↑↑ | ↑↑ | No | 107 |
| Bmp6 | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑↑ (M) → | |||
| Bmp6 × Tfr2 | Tfr2 | ↓↓↓ | ↑ | ↑↑ | 107 | |
| Bmp6 | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑ (M) → | |||
| Hjv × Hfe | Hfe | ↓↓ | ↑↑↑ | n/a | No | 10,106 |
| Hjv | → | → | n/a | |||
| Hjv × Tfr2 | Tfr2 | → | ↑ | n/a | No | 105,106 |
| Hjv | → | → | n/a | |||
| Hfe × Tfr2 | Hfe | ↓↓ | ↑↑ | n/a | No | 97 |
| Tfr2 | ↓ | ↑ | n/a | |||
| Acvr2a × Bmpr2* | Acvr2a* or Bmpr2* | ↓↓↓ | ↑↑↑↑ | n/a | No | 69 |
| Smad1 × Smad5* | Smad1* or Smad5* | ↓↓↓ | ↑↑↑↑ | n/a | No | 70 |
| Tmprss6 × Hfe | Tmprss6 | → | → | → | Yes | 116 |
| Hfe | ↑ | ↓ | ↓ | |||
| Tmprss6 × Hjv | Tmprss6 | ↓↓↓ | ↑↑↑↑ | ↑↑ | No | 109 |
| Hjv | → | → | → | |||
| Tmprss6 × Tfr2 | Tmprss6 | → | → | n/a | Yes | 109,117 |
| Tmprss6 × Bmp6 | Tmprss6 | ↓ | ↑ | → | No | 149 |
| Bmp6 | ↑↑ | ↓↓ | ↓ |
| Double mutant . | Compared with . | Hamp levels . | Liver iron . | Extrahepatic iron loading . | Anemia . | References . |
|---|---|---|---|---|---|---|
| Bmp6 × Hjv | Bmp6 or Hjv | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑↑ (M) → | No | 90,107 |
| Bmp6 × β2m | β2m | ↓↓↓ | ↑↑ | ↑↑ | No | 107 |
| Bmp6 | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑↑ (M) → | |||
| Bmp6 × Tfr2 | Tfr2 | ↓↓↓ | ↑ | ↑↑ | 107 | |
| Bmp6 | (F) ↓↓↓ (M) ↓↓ | → | (F) ↑ (M) → | |||
| Hjv × Hfe | Hfe | ↓↓ | ↑↑↑ | n/a | No | 10,106 |
| Hjv | → | → | n/a | |||
| Hjv × Tfr2 | Tfr2 | → | ↑ | n/a | No | 105,106 |
| Hjv | → | → | n/a | |||
| Hfe × Tfr2 | Hfe | ↓↓ | ↑↑ | n/a | No | 97 |
| Tfr2 | ↓ | ↑ | n/a | |||
| Acvr2a × Bmpr2* | Acvr2a* or Bmpr2* | ↓↓↓ | ↑↑↑↑ | n/a | No | 69 |
| Smad1 × Smad5* | Smad1* or Smad5* | ↓↓↓ | ↑↑↑↑ | n/a | No | 70 |
| Tmprss6 × Hfe | Tmprss6 | → | → | → | Yes | 116 |
| Hfe | ↑ | ↓ | ↓ | |||
| Tmprss6 × Hjv | Tmprss6 | ↓↓↓ | ↑↑↑↑ | ↑↑ | No | 109 |
| Hjv | → | → | → | |||
| Tmprss6 × Tfr2 | Tmprss6 | → | → | n/a | Yes | 109,117 |
| Tmprss6 × Bmp6 | Tmprss6 | ↓ | ↑ | → | No | 149 |
| Bmp6 | ↑↑ | ↓↓ | ↓ |
Levels of hepcidin expression and tissue iron loading are subject to variation based on background strains ages, and analytical variations in different studies.
Hepatocyte conditional knockout.
Hepcidin regulation by HFE and TFR2
Like mutations in HJV or hepcidin, mutations in HFE or TFR2 also lead to HH, although with less severe iron overload that presents later in life.43 HFE is an atypical major histocompatibility complex class I-like molecule.93 TFR2 is a receptor for the plasma iron carrier transferrin with more limited tissue distribution and a lower affinity than TFR1, the primary receptor for transferrin-bound iron uptake.94 Both HFE and TFR2 stimulate hepcidin expression through a functional interaction with the SMAD signaling pathway (Figure 2), as demonstrated by blunted SMAD signaling and hepcidin expression in mice and/or patients with mutations in these genes.95-100 In vitro overexpression systems demonstrated that HFE and TFR2 bind to each other101 and to HJV,91 and these protein interactions are thought to be one mechanism by which HFE and TFR2 influence hepcidin production. HFE was also demonstrated to bind ALK3 and increase its stability by interfering with ubiquitination and degradation.102 Whether these protein interactions occur with endogenously expressed proteins in vivo remains uncertain. Notably, genetic studies suggest that the functions of these proteins are not entirely overlapping and that some proteins have a more dominant role than others (Tables 1 and 2). For example, combined knockout/mutation of HFE and TFR2 resulted in more severe iron overload than single knockout/mutation of either gene in both mice and humans.97,103 Moreover, Tfr2 mutant mice overexpressing an Hfe transgene in hepatocytes had hepcidin excess and iron deficiency, suggesting that HFE can induce hepcidin in the absence of TFR2.104 Hjv knockout mice had more severe iron overload than Hfe or Tfr2 knockout mice (similar to the human phenotype43 ) and were not worsened by a combined loss.105,106 Knockout of Hjv, Tfr2, or β2 microglobulin (a chaperone required for HFE localization) further dampened SMAD signaling, hepcidin expression, and iron overload in Bmp6 knockout mice,107 suggesting that these proteins can modulate hepcidin using BMP2 (or other BMP ligands) in the absence of BMP6. Taken together, the current data suggest that HJV, HFE, and TFR2 all converge on the same pathway to modulate BMP-SMAD signaling and thereby hepcidin. Although all proteins must be present for optimal signaling, multiple types of BMP ligand/receptor/accessory protein complexes appear to be capable of stimulating hepcidin production to some degree, and none of the single protein components are absolutely required, so loss of any one protein results in a modulatory effect rather than a complete abolishment of signaling. HFE and/or TFR2 may also have additional roles outside of modulating the BMP-SMAD pathway to regulate hepcidin production. For example, TFR2 is also expressed in erythroid cells, where it has a functional role to modulate erythropoietin (EPO) signaling and erythropoiesis according to iron availability108,109 and therefore may also contribute to hepcidin regulation via the erythroid signal.108,109
Hepcidin regulation by TMPRSS6
Mutations or knockout of TMPRSS6 result in IRIDA, characterized by hepcidin excess and iron deficiency anemia that does not respond to oral iron and only partially responds to parental iron.53-55 Common variants in TMPRSS6 are also associated with alterations in iron and red blood cell parameters.110,111 A serine protease that is highly expressed in the liver, TMPRSS6 (also known as matriptase-2), also appears to regulate hepcidin by modulating the central BMP-HJV-SMAD signaling pathway (Figure 2). In particular, Tmprss6 knockout mice exhibited increased SMAD signaling in parallel to hepcidin excess, and ablation of Tmprss6 in Hjv knockout mice did not reverse hepcidin deficiency or iron loading112 (Table 2). The mechanism of action of TMPRSS6, as demonstrated by in vitro overexpression systems, is to bind and cleave HJV, thereby releasing the coreceptor from the membrane surface and blunting BMP-SMAD signaling.92 Although recombinant soluble HJV fused to immunoglobulin G Fc inhibited BMP-SMAD signaling by sequestering BMP ligands from the receptor signaling apparatus,82 TMPRSS6-cleaved HJV did not inhibit signaling.113 Interestingly, Tmprss6 knockout mice exhibited reduced rather than increased liver HJV expression,114 as might be anticipated based on its proposed mechanism of action. Recently, TMPRSS6 overexpression in hepatocytes was demonstrated to reduce hepcidin expression in Hjv knockout mice, suggesting the existence of another mechanism for TMPRSS6-mediated hepcidin suppression independent of HJV.115 In this study, TMPRSS6 was also shown to cleave other members of the BMP-SMAD signaling apparatus, including ALK3, ALK2, ACVR2A, BMPR2, HFE, TFR2, and neogenin, in in vitro overexpression systems.115 Nevertheless, HFE and TFR2 do not appear critical to TMPRSS6 function in vivo, since mice with a combined deletion of Tfr2 and/or Hfe and Tmprss6 still exhibited a similar degree of hepcidin excess and iron deficiency anemia as single Tmprss6 knockout mice109,116,117 (Table 2). A limitation for most of these studies is the use of an overexpression system to ascertain the molecular targets of TMPRSS6. The identification of TMPRSS6 as an endogenous hepcidin suppressor has resulted in the development of novel TMPRSS6-inhibiting therapies that have shown promise for increasing hepcidin to limit iron overload in mouse models of β-thalassemia and HH.118,119
Iron sensing in the liver
There are 2 distinct iron signals that regulate hepatocyte hepcidin production: acute changes in plasma iron and changes in tissue iron.39,40 Both of these iron signals stimulate hepcidin production by activating the BMP-SMAD signaling cascade, but through different mechanisms (Figure 2). Tissue iron is sensed by liver endothelial cells to regulate production of BMP ligands, which have paracrine effects to induce hepcidin production in hepatocytes. Indeed, chronic dietary iron loading or deficiency that alters liver iron levels concordantly regulated Bmp6 and Bmp2 messenger RNA (mRNA) expression in mouse liver endothelial cells,66,120 although Bmp6 expression was modulated more than Bmp2.66 BMP6 regulation by iron appears to be a cell-autonomous event in liver endothelial cells, since Bmp6 was induced more strongly by dietary iron in Zip14/Slc39a14 knockout mice that fail to load iron in hepatocytes but load more iron in liver nonparenchymal cells,121 and importantly, Bmp6 was induced by iron loading in isolated liver endothelial cell primary cultures.122 One mechanism by which iron induces BMP6 in liver endothelial cells is through transcriptional regulation by NRF2, which is activated by iron-induced reactive oxygen species.123 NRF2 activation in this context has the dual benefit of inducing other antioxidant protective pathways in addition to limiting further iron loading as a consequence of BMP6-mediated hepcidin upregulation.121
Acute increases in plasma iron in the absence of liver iron loading also induce hepcidin expression, but without inducing liver Bmp6 mRNA expression.39 This is associated with an induction of the downstream SMAD1/5/8 signaling cascade.39 Whether SMAD1/5/8 activation in this context occurs downstream of BMP6 or in response to BMP2 or other BMP ligands is still not known. Hepatocyte HFE and TFR2 are both proposed to play a critical role in sensing plasma iron levels in the form of iron-bound transferrin (holo-transferrin). TFR2 binds directly to holo-transferrin.94 HFE binds to transferrin receptor 1 (TFR1) at the transferrin binding site and is displaced by increased holo-transferrin.124,125 Interestingly, mice expressing mutant TFR1 that does not bind HFE exhibited high hepcidin.126 Thus, displacement of HFE by holo-transferrin may be one mechanism by which hepatocytes sense plasma iron levels to upregulate hepcidin.126 Cell culture models suggest holo-transferrin favors the interaction between HFE and TFR2 and this may also play an important role in hepcidin induction by iron.101 Notably, iron reduces TFR1 and increases TFR2 levels, which may also help favor the release of HFE from TFR1 and/or interaction with TFR2. For TFR1, mRNA stability is reduced by intracellular iron loading through the iron regulatory protein (IRP)/iron response element (IRE) system, which is key mediator of cellular iron homeostasis (discussed below).2 In contrast, TFR2 is stabilized by holo-transferrin,127,128 which redirects it from a degradation to a recycling pathway.129 Holo-transferrin also reduces release of TFR2 from the membrane surface.130
Additional mechanisms may also be involved in sensing iron deficiency (Figure 2). A low-iron diet and cellular iron deficiency increase TMPRSS6 by inhibiting its degradation.131-133 Iron deficiency also increases HJV cleavage by the furin family of proprotein convertases,134-136 generating a distinct form of soluble HJV (sHJV) compared with TMPRSS6 cleavage. Circulating sHJV was increased in mice on a low-iron diet, consistent with increased HJV cleavage by one or both of these mechanisms.137 Thus, iron deficiency may suppress hepcidin production by release of membrane-bound HJV from hepatocytes. Furin-cleaved sHJV has also been demonstrated to act as a decoy to inhibit BMP-mediated hepcidin induction, although this property is not shared by TMPRSS6-cleaved sHJV.113 However, circulating sHJV levels in vivo are relatively low137 and may not be sufficient to inhibit BMP signaling.
Hepcidin regulation by inflammation, erythropoietic demand, and other stimuli
Hepcidin induction by inflammation plays a protective role against some infections by lowering available circulating iron138,139 ; however, excessive hepcidin induction in chronic inflammatory diseases contributes to iron restricted erythropoiesis and anemia.41 Mechanistically, inflammation upregulates hepcidin predominantly by interleukin-6,140 which induces phosphorylation of signal transducer and activator of transcription 3 that binds to the hepcidin promoter adjacent to the proximal BMP response element.141-143 Other inflammatory cytokines such as interleukin-1β and activin B may also contribute, although their functional roles in vivo are less clearly defined.41
Erythropoietic drive due to hypoxia, anemia, or pathologic conditions of ineffective erythropoiesis induce the kidney to produce EPO, which not only increases proliferation and terminal differentiation of red blood cells but also induces erythroblasts to secrete erythroferrone (ERFE) and possibly other “erythroid regulators” that function to suppress hepcidin.2 This not only ensures the adequate delivery of iron to support erythropoiesis but also contributes to pathologic iron loading in conditions of ineffective erythropoiesis, such as in β-thalassemia. A key role for ERFE was demonstrated by the failure of Erfe knockout mice to suppress hepcidin in response to hemorrhage or EPO injection144 and the amelioration of hepcidin deficiency and iron loading in a Erfe knockout thalassemia model.145 Other proposed erythroid regulators include platelet-derived growth factor BB, which is induced by hypoxia in humans and mice and suppresses hepcidin when injected into mice.146 Growth and differentiation factor 15 is induced in the blood of patients with thalassemia,147 but its functional role in hepcidin suppression is less certain.2
Hepcidin regulation by erythropoietic drive and inflammation depend on a functional SMAD signaling pathway, suggesting important cross talk with the iron-sensing pathway. EPO and ERFE suppressed liver SMAD1/5 phosphorylation in wild-type mice and primary hepatocytes, respectively, and failed to suppress hepcidin in hepatocyte Smad1/5 conditional knockout mice.70 EPO likely suppresses the SMAD1/5 pathway at least in part by increasing iron consumption for erythropoiesis, reducing circulating holo-transferrin, and triggering the liver iron sensing pathway.148,149 The mechanism of action of ERFE to suppress SMAD1/5 signaling and hepcidin directly is less certain. Its effects do not require BMP6 or HJV, since EPO still suppressed hepcidin in Bmp6 and Hjv knockout mice.149,150 Its direct effects are also independent of TMPRSS6, since ERFE efficiently suppressed hepcidin in Tmprss6 knockout primary hepatocytes.151 However, Tmprss6 knockout mice failed to suppress hepcidin in response to EPO despite ERFE induction, similar to mice on a high-iron diet, suggesting that ERFE’s actions in vivo are impaired when SMAD signaling is high.149 Notably, Tmprss6 inhibition ameliorated hepcidin deficiency and iron overload in a thalassemia mouse model, demonstrating the overall importance of TMPRSS6 function for erythroid-mediated hepcidin suppression in conditions of ineffective erythropoiesis with potential therapeutic implications.118,119,152
The cross talk between inflammation and BMP-SMAD signaling in hepcidin regulation is demonstrated by blunted hepcidin induction by inflammation in hepatocyte conditional Smad4 knockout mice,63 when the hepcidin promoter proximal BMP response element was mutated,153 or in the presence of BMP inhibitors.84,85 This cross talk is postulated to occur at the level of the hepcidin promoter or by stimulating production of the BMP/transforming growth factor-β superfamily member activin B, which can use BMP type I receptors to induce SMAD1/5/8 phosphorylation and hepcidin expression in hepatocytes.89,154 However, a functional role for activin B in vivo is uncertain given preserved hepcidin induction by inflammation in activin B knockout mice.155
In addition to inflammation and erythropoietic drive, a variety of other stimuli have been demonstrated to regulate hepcidin production, including endoplasmic reticulum stress, hormones (testosterone, estrogen, and progesterone), growth factors (hepatocyte growth factor and epidermal growth factor), and other signaling pathways (Ras/RAF and mTOR).156,157 Many of these mediators also cross talk with the central BMP-SMAD pathway.156,158
Cross talk between systemic and cellular iron homeostasis
Iron sensing at the cellular level in nonhepatic tissues involved in systemic iron transport and erythropoiesis can also contribute to systemic iron homeostasis. A key mediator of intracellular iron homeostasis is the IRP/IRE system.2 In iron-deficient conditions, IRPs bind to IREs in the 3′ untranslated region of transcripts such as TFR1 and DMT1 to increase mRNA stability or in the 5′ untranslated region of transcripts such as ferritin or ferroportin to inhibit translation.2 IRPs are inactivated in iron-replete conditions either by binding to an iron sulfur cluster or by being targeted for proteosomal degradation by the iron-binding hemerythrin and E3-ubiqutin ligase F-box/leucine-rich repeat protein 5.2 Another sensor of cellular iron status is the hypoxia-inducible factor (HIF) family of transcription factors that are stabilized to regulate gene transcription by iron deficiency or hypoxia. HIFs are inactivated in iron-replete conditions by the oxygen- and iron-dependent prolyl hydroxylases that target regulatory α subunits for degradation.1 A prime example where cellular iron homeostasis can influence systemic iron homeostasis is duodenal enterocytes, where HIF-2α mediates upregulation of DMT1, duodenal cytochrome B, and ferroportin in response to iron deficiency to influence duodenal iron uptake.159-161 Indeed, genetic disruption of intestinal HIF-2α signaling reduced serum and tissue iron levels and exacerbated iron deficiency and anemia by a low-iron diet despite appropriate hepcidin suppression.159,160 IRP1 appears to have more of a counterregulatory effect in this system by downregulating HIF-2α.162
Conclusions
The hepcidin-ferroportin axis is a key mediator of systemic iron homeostasis by controlling iron availability from dietary sources and body stores to support erythropoiesis and other essential functions but prevent the toxicity of iron excess. Insufficient or excess activity of this pathway contributes to most disorders of iron metabolism, including HH, iron-loading anemias, anemia of inflammation, and IRIDA. Identifying the genetic basis of iron disorders has provided important advances in understanding how the liver senses plasma and tissue iron levels and integrates these signals with inflammation and erythropoietic drive to control hepcidin production. Future research is needed to fully comprehend the molecular mechanisms of iron sensing and the complex interplay among the proteins involved in hepcidin regulation. These studies are already contributing to the development of novel therapies for iron disorders.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant RO1-DK087727) (J.L.B.).
Authorship
Contribution: J.L.B. wrote the manuscript and edited the figures and tables; and C.-Y.W. created the figures and tables and edited the manuscript.
Conflict-of-interest disclosure: J.L.B. has ownership interest in Ferrumax Pharmaceuticals and has received consulting fees from Keryx Biopharmaceuticals and Disc Medicine. C.-Y.W. declares no competing financial interests.
Correspondence: Jodie L. Babitt, Division of Nephrology, Program in Membrane Biology, Center for Systems Biology, Massachusetts General Hospital, 185 Cambridge St, CPZN-8208, Boston, MA 02114; e-mail: babitt.jodie@mgh.harvard.edu


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal